Now available: Panorama™ vanishing twin workflow
Learn MoreNow available: Panorama™ vanishing twin workflow Learn More
The only SNP-based NIPT
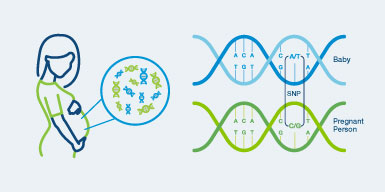
Only Panorama™
Panorama™ is the only SNP-based NIPT able to distinguish between the pregnant person’s and fetal (placenta) DNA to deliver unique, clinically validated capabilities.1-6

Validated with clinical rigor
Demonstrated performance in a real-world population in the largest prospective NIPT study. 99% sensitivity, >99% specificity, and 95% positive predictive value (PPV) for trisomy 211-4 Panorama™ had zero fetal sex errors in validation studies*2-4,7

Continuously innovating
Enhancing test capabilities for 10+ years
Detects conditions that other tests cannot, including maternal X mosaicism, triploidy and vanishing twin.8,9,10
High-quality screening for all pregnancies
Comprehensive support services
NateraCore supports busy clinicians and their patients throughout the testing journey with resources accessible anytime, anyplace.
- Patient-friendly multi-language educational materials
- Complimentary pre- and post-test information sessions with board-certified genetic counselors
- Clear, actionable reports served with time-saving tools and access to expert guidance
- Value added services that go beyond the test to address what’s next

Complete Test Specifications for Singleton, Egg Donor, Gestational Carrier, and Monozygotic (Identical) Twins
Definition of performance terms
Sensitivity is the ability to correctly identify a truly high risk case as high risk. For example, in a group of Trisomy 21 cases, Panorama will correctly identify more than 99% of those cases.
Specificity is the ability to correctly identify an unaffected case as low risk.
Positive Predictive Value (PPV) is the likelihood the result says high risk and the fetus is actually affected. For example, when Panorama shows a high risk result for Trisomy 21, there is a 95% chance that the fetus is affected by Trisomy 21. In other words, 5% of the time, you could get a high risk result when the fetus is not affected by Trisomy 21.
Negative Predictive Value (NPV) is the likelihood the result says low risk and the fetus is truly not affected.
| Condition | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV |
|---|---|---|---|---|
| Trisomy 21*16,17 | 99.0% (CI 97.1-100) | >99% (CI 99.93-99.99) | 95% | >99.99%‡ |
| Trisomy 18*16,17 | 94.1% (CI 82.9-100) | >99% (CI 99.96-100) | 91% | >99.99%‡ |
| Trisomy 13*16,17 | >99% (CI 73.5-100) | >99% (CI 99.96-100) | 68% | >99.99%‡ |
| Monosomy X**17,18 | 83.3% (CI 53.5-100) | >99% (CI 99.7-100) | 78% | >99.99%‡ |
| Triploidy**9,19 | >99% (CI 66.4-100) | >99% (CI 99.5-100) | 7.5% | >99.99%‡ |
| XXX, XXY, XYY**20 | 73.1% (CI 61.0-85.1) | 99.9% (CI 99.90-99.99) | 83% | 99.87%‡ |
| 22q11.2 deletion syndrome**1 | 83.3% (CI 51.6-97.9) | >99% (CI 99.91-99.98) | 53% | 99.9%||(CI 99.9-100) |
| 1p36 deletion syndrome**21,22 | >99% (CI 2.5-100) | >99% (CI 99.1-100) | 7-17%|| | 99.98-99.99%|| |
| Angelman syndrome**†21,22 | 95.5% (CI 77.2-99.9) | >99% (CI 99.1-100) | 10%§ | >99.99% |
| Cri-du-chat syndrome**21,22 | >99% (CI 85.8-100) | >99% (CI 99.1-100) | 2-5%|| | >99.99% |
| Prader-Willi syndrome**†21,22 | 93.8% (CI 69.8-99.8) | >99% (CI 99.1-100) | 5% | >99.99% |
| Female | >99.9% (CI 99.4-100) | >99.9% (CI 99.5-100) | ||
| Male | >99.9% (CI 99.5-100) | >99.9% (CI 99.4-100) |
Test Specifications for Dizygotic (Nonidentical) Twins23
| Condition | PPV |
|---|---|
| Trisomy 21 | 88% |
| Trisomy 18 | 70% |
| Trisomy 13 | 67% |
More than 10% of dizygotic pregnancies may not receive a result due to low fetal fractions.7
Is Panorama™ right for you?
We’re here to help you find out
References
1Dar et al. Am J Obstet Gynecol. Epub prior to publication. https://doi.org/10.1016/j.ajog.2022.01.002
2Pergament et al. Obstet Gynecol. 2014 Aug; 124(2 Pt 1):210-8.
3Nicolaides et al. Prenat Diagn. 2013 June; 33(6):575-9.
4Ryan et al. Fetal Diagn Ther. 2016; 40(3):219-223.
5ACOG Practice Bulletin 226. Obstet Gynecol. 2020 Oct; 136(4):859-867.
6Natera internal data on file as of Sept 2023.
7Norwitz et al. J Clin Med. 2019 Jun; 8:937.
8Martin KA et al. Am J Obstet Gynecol MFM. 2020; 2:100152.
9Kantor V, et al. Prenat Diagn. 2022 Jul; 42(8):994-999.
10Curnow KJ, et al. Am J Obstet Gynecol. 2015 Jan; 212(1):79.e1-9.
11Hedriana et al. Prenat Diagn. 2020 Jan; 40(2):179-84.
12Society for Maternal-Fetal Medicine, Clinical guideline: Twin-twin transfusion syndrome, Jan 2013.
13Oldenburg et al. Ultrasound Obstet Gynecol. 2012 Jan; 39(1); 39:69–74.
14Chasen, Chervenak (2017). Twin pregnancy: Prenatal issues. In T. Post (Ed.), UpToDate. Waltham, Mass.: UpToDate. Retrieved from www.uptodate.com
15Cunningham et al. Williams Obstetrics. 24th edition. New York: McGraw-Hill Education, 2014.
16Dar et al. Am J Obstet Gynecol. 2022 Aug; 227(2):259.e1-259.e14.
17DiNonno et al. J Clin Med. 2019 Aug 26; 8(9):1311.
18Martin et al. Genet in Med. 2023;25(8):100879
19Nicolaides et al. Fetal Diagn Ther. 2014. 35;(3):212-7.
20Martin K et al. Genet in Med. 2023. https://doi.org/10.1016/j.gim.2023.100879
21Martin et al. Clin Genet. 2018 Feb; 93(2):293-300.
22Wapner et al. Am J Obstet Gynecol. 2015 Mar; 212(3):332.e1-9.
23Kantor V, et al. Prenat Diagn. 2022 Dec; 42(13).
Footnotes
* CA residents: If your clinician ordered screening through the California Prenatal Screening program using Natera’s Vasistera™ NIPT, Panorama™ will only screen for supplemental conditions. If this is the case, trisomies 21, 18 and 13, and fetal sex (optional) will be screened using Vasistera™ NIPT and will be reported separately.
** Not available for egg-donor or gestational carrier pregnancies or in cases of dizygotic (nonidentical) twins. Triploidy and microdeletions except for 22q11.2 deletions are not available for monozygotic (identical) twins.
† This test has been validated on full region deletions of Prader-Willi syndrome/Angelman syndrome (PWS/AS) only and might be unable to detect smaller deletions. It has not been validated for other molecular mechanisms which could cause PWS/AS such as uniparental disomy (UPD) or methylation.
‡ Ongoing clinical follow-up is performed to ensure the NPV does not fall below the quoted value but follow up is not obtained for all low risk calls.
§ PPV for 22q11.2 deletion syndrome and Angelman syndrome in published studies was 53% and 10% respectively when no ultrasound anomalies were seen and was up to 100% when ultrasound anomalies were seen prior to testing.
|| Dependent upon fetal fraction (FF). For 22q11.2 deletion syndrome, only the paternal allele is evaluated at FF ≤ 6.5%. For 1p36 deletion syndrome and Cri-du-chat syndrome, only the paternal allele is evaluated at FF < 7%. For Angelman syndrome, no risk assessment is reported at FF < 7%. For Prader-Willi syndrome, no risk assessment is reported at FF ≤ 2.8%.