DNA is in our blood
Natera™ is a global leader in cell-free DNA (cfDNA) testing, dedicated to oncology, women’s health, and organ health. We aim to make personalized genetic testing and diagnostics part of the standard of care to protect health and inform earlier, more targeted interventions that help lead to longer, healthier lives.

Innovating with Integrity
Validation and Evidence
We validate our tests with clinical studies and make data publicly available.
>250
peer-reviewed publications
Quality and Accuracy
We meet or exceed applicable industry and regulatory standards for all our tests.
CLIA-certified/
CAP–accredited laboratories
Support and Transparency
We provide expert support to help make our test results clear and easy to understand.
>70K
patient/provider support sessions annually
Investing in Research
We have participated in some of the largest cfDNA prospective trials conducted to date.
- CIRCULATE : A study of molecular-residual-disease-guided treatment in 3,000+ patients with colorectal cancer using the Signatera™ personalized tumor-informed circulating tumor DNA assay
- SMART: The largest prospective, real-world population study of noninvasive prenatal testing (NIPT) conducted to date, involving 18,000+ pregnancies
- Trifecta: The largest prospective, fully biopsy-matched cohort study to date evaluating donor-derived cell-free DNA (dd-cfDNA) for kidney transplant recipients

Reporting Results with Context
We provide clear and detailed test reports to help inform next steps.
- Signatera™: reports whether circulating tumor DNA (ctDNA) is detected and provides the mean tumor molecular per ML (MTM/mL) for longitudinal assessment
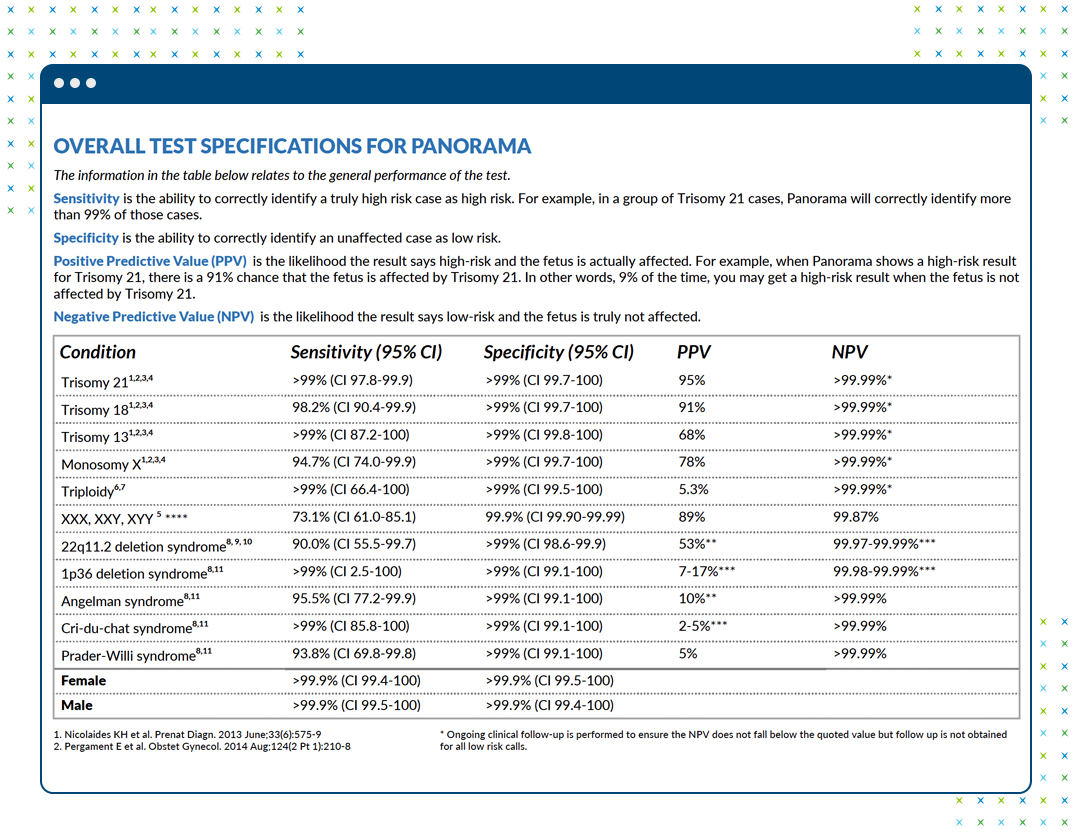
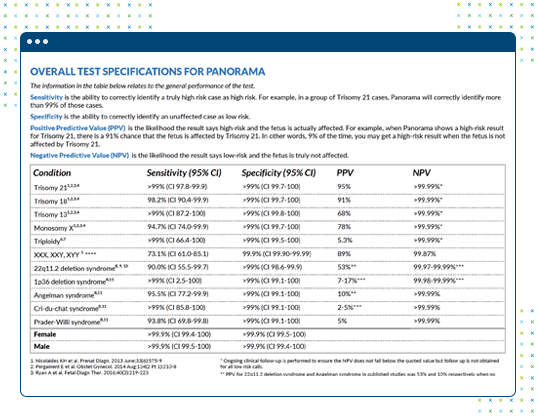
- Panorama™: reports positive predictive value (PPV) for high risk results, negative predictive value (NPV) for low risk results, and a fetal fraction assessment
- Prospera™: reports rejection through analysis of donor-derived cfDNA
Acting with Care for the Future
We are committed to reducing the environmental impact of our business.
- Natera complies with all applicable local, state, and federal environmental regulations and we conduct regular reviews to ensure our supplier partners and contractors do the same.
- As part of our sustainability program, we are committed to reducing the environmental impact of our laboratories, our corporate offices, our supply chain, and our waste operations.

Advancing the leading edge of molecular diagnostics
Natera was founded from a personal mission to help change standards of care.

In 2004, my sister gave birth to a son with a severe genetic condition. He passed away six days after birth. It was a devastating experience for our entire family. I founded Natera because I believe all families deserve access to technologies that offer early detection of genetic disease.