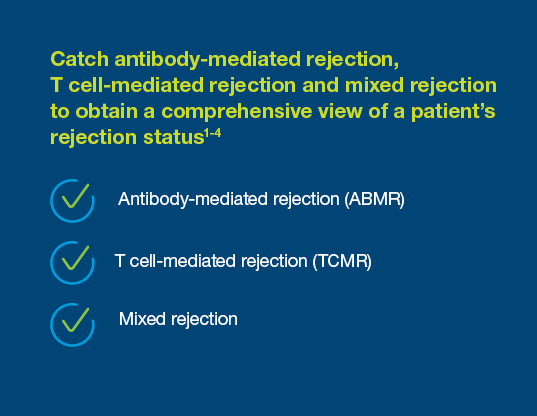
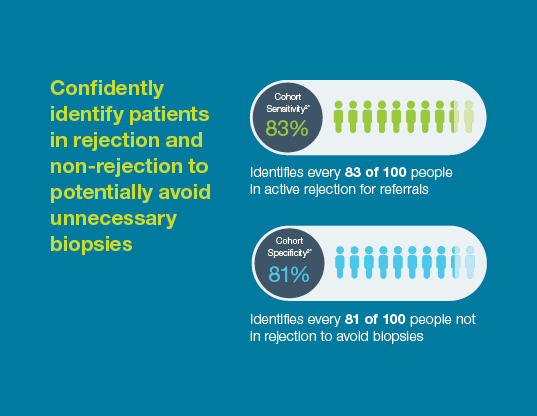
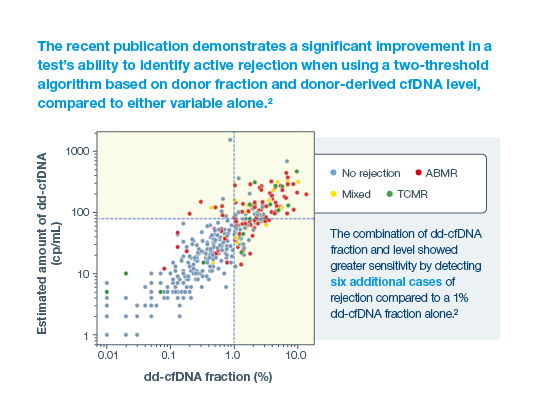
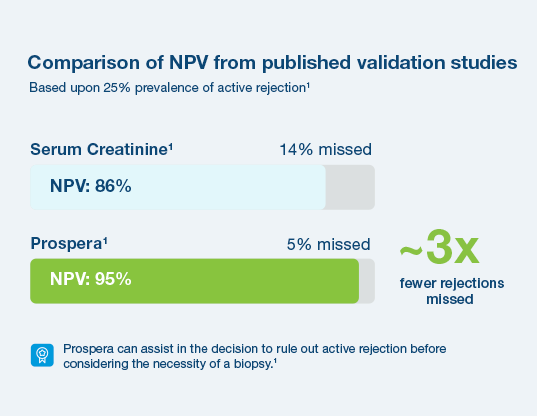
Prospera offers more precise post-transplant rejection assessment
With a 95% negative predictive value, Prospera misses ~3x fewer rejections than serum creatinine.1 Prospera’s proprietary donor-derived cell-free DNA (dd-cfDNA) technology offers early warning signs of transplant rejection and reduces the likelihood of renal allograft failure with a non-invasive single blood draw.1,2
Why now?
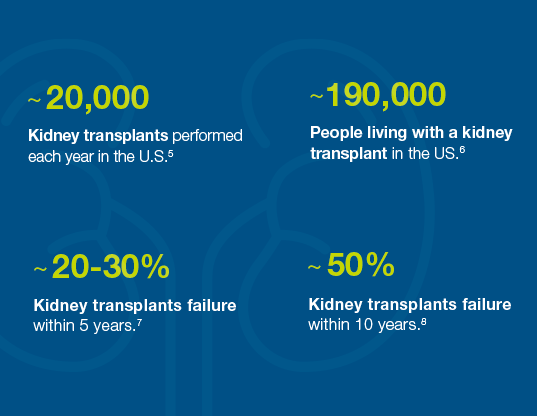
Organ rejection is a problem
Many kidney transplant failures occur within the first five to ten years7,8 because organ rejection isn’t caught early enough or treated effectively. As a result, the patient needs a new organ and must start the waitlist process again.
Why Choose Prospera™ for Rejection Assessment?
Built on our deep experience and legacy in cell-free DNA, Prospera™ is:
Access Prospera through our remote service for routine labs
Find out more about Prospera for Kidney transplant recipients
References
1Sigdel TK, Archila FA, Constantin T, et al. Optimizing detection of kidney transplant injury by assessment of donor derived cell-free DNA via massively multiplex PCR. J Clin Med. 2018;8(1):pii E19
2Halloran, PF. et al. Combining Donor-derived Cell-free DNA Fraction and Quantity to Detect Kidney Transplant Rejection Using Molecular Diagnoses and Histology as Confirmation. Transplantation: June 29, 2022 - doi: 10.1097/TP.0000000000004212
3Bunnapradist, S. Using both fraction and quantity of donor-derived cell-free DNA to detect kidney allograft rejection, J Amer Soc Nephrology 2021, in press
4Lum et al. Single center experience comparing two clinically available donor derived cell free DNA tests and review of literature, Transplantation. 2021 doi.org/10.1016/j.tpr.2021.100079
5Organ Donation Statistics. U.S. Department of Health and Human Services. U.S. Government Information on Organ Donation and Transplantation. https://www.organdonor.gov/statistics-stories/statistics.html. Published March 31, 2016.
6Kidney Disease Statistics for the United States. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Published Dec. 1, 2016.
7Stegall et al. Through a Glass Darkly: Seeking Clarity in Preventing Late Kidney Transplant Failure, J Am Soc Nephrol. 2015; 26 (1):20-9
8Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal, Am J of Transplantation. 2011; Mar;11(3):450-62.5.
9Bunnapradist, S. Using both fraction and quantity of donor-derived cell-free DNA to detect kidney allograft rejection, J Amer Soc Nephrology 2021, in press