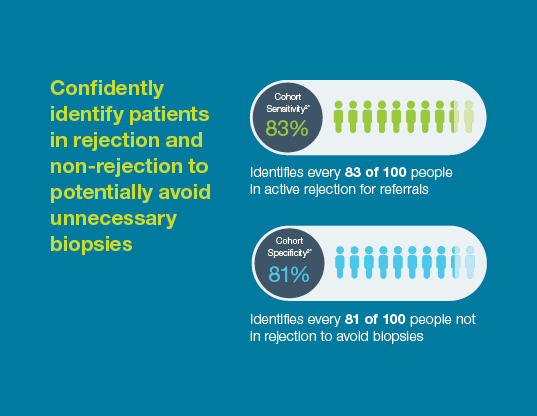
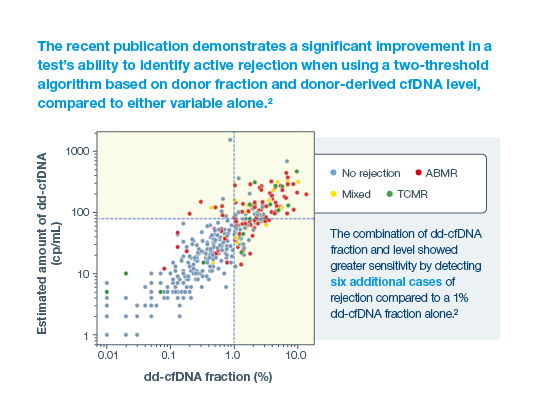
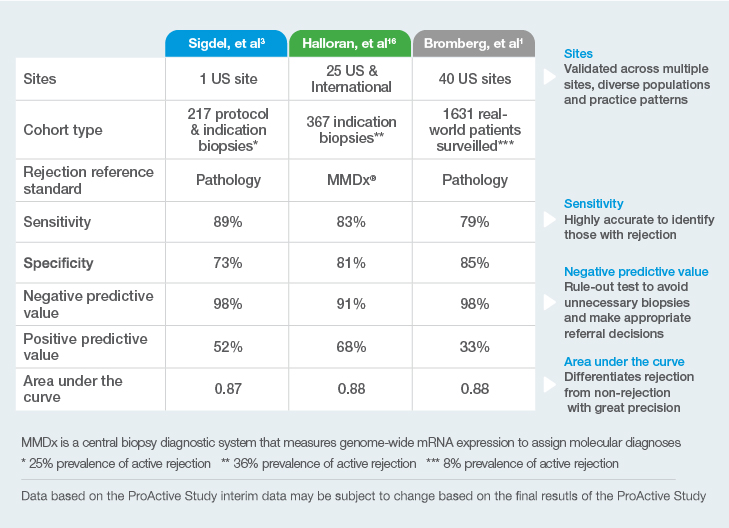
Why is the Prospera™ test a proven leading indicator of rejection?
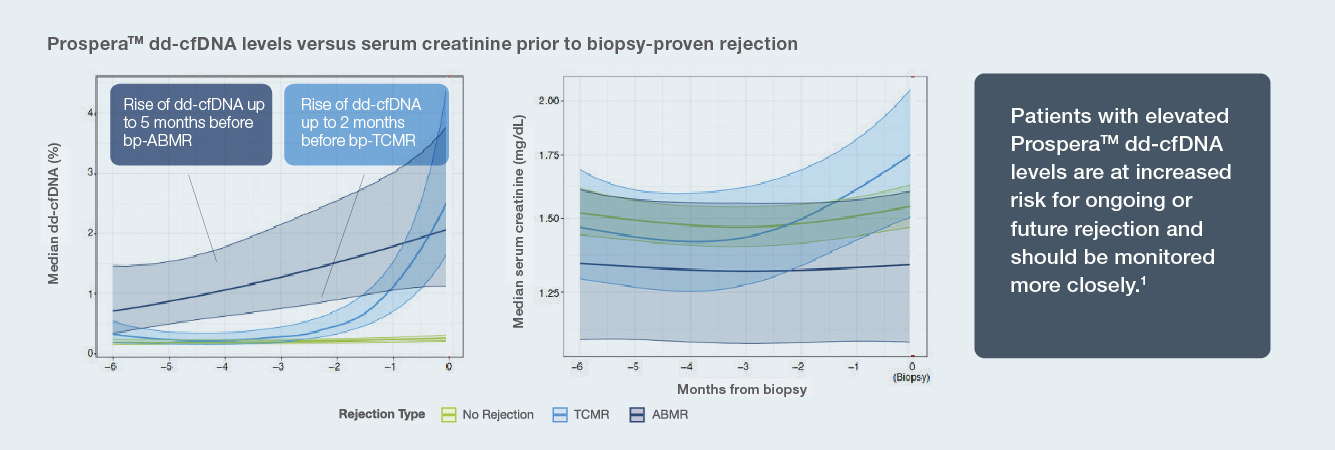
Published findings from the ProActive Study showcased Prospera™ donor-derived cell-free DNA (dd-cfDNA) test predicted antibody-mediated rejection up to five months and T cell-mediated rejection up to two months in advance of biopsy proven rejection.
With 54 participating transplant centers, the ProActive Study is the largest prospective dd-cfDNA registry in kidney transplant recipients.
What is the Prospera™ Test?

Prospera™ is a transplant rejection test that uses a simple blood draw to assess the risk of rejection of a transplanted kidney.
Through the use of advanced cell-free DNA technology, Prospera™ increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help providers develop a treatment plan to best protect the donated kidney.
Why Choose Prospera™ for Rejection Assessment?
Built on our deep experience and legacy in cell-free DNA, Prospera™ is:
Refined Workflow. Only from Natera
Natera has performed over five million cell-free DNA (cfDNA) tests across women’s health and oncology. Harnessing this expertise, only Prospera™ uses one-of-a-kind, third-generation cfDNA technology to differentiate between self cfDNA and cfDNA from another source—such as a donated organ.

Hear What Your Colleagues are Saying
Incorporating Prospera™ into your Workflow

Clinician’s Guide to Results
Natera’s clinical staff provides direct support to help you understand how to use the Prospera test and your patients’ results.
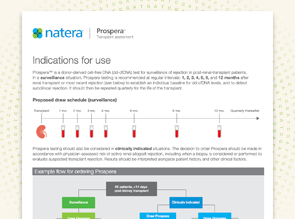
Indications for Use
View the proposed Prospera draw schedule in surveillance and clinically-indicated scenarios

Why Prospera
Introducing our series of educational content on how our Prospera transplant assessment tool can help transplant physicians provide better patient care in specific clinical scenarios.

Managing your Prospera Orders & Results
Our Organ Health provider portal easily allows you to order, track, view, renew, and share your patients’ Prospera results.
Is Prospera™ right for you?
We’re here to help you find out
1Bromberg, et al; on behalf of the ProActive Investigators. Elevation of Donor-derived Cellfree DNA Before Biopsy-proven Rejection in Kidney Transplant. Transplantation ():10.1097/TP.0000000000005007, April 08, 2024. | DOI: 10.1097/TP.0000000000005007
2Halloran, PF. et al. Combining Donor-derived Cell-free DNA Fraction and Quantity to Detect Kidney Transplant Rejection Using Molecular Diagnoses and Histology as Confirmation. Transplantation: June 29, 2022 – doi: 10.1097/TP.0000000000004212
3Sigdel TK et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med. 2019;8(1):19.