Don’t miss pregnancies affected by 22q11.2 deletion syndrome
Choose high detection screening with Panorama™, including the highest published clinical sensitivity1
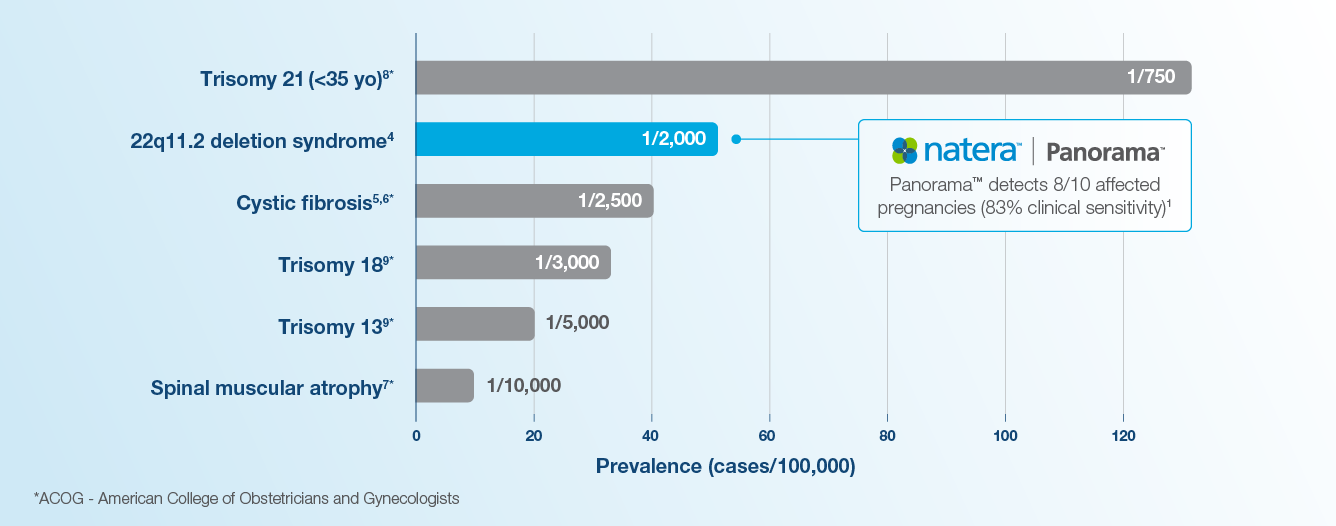
22q (DiGeorge Syndrome) is more common than most ACOG recommended conditions
Maternal age is not a risk factor for having a pregnancy affected with 22q.1-9

WEBINAR
22q11.2 Deletion Syndrome (DiGeorge):
Two cases, two outcomes. The importance of prenatal identification
60 minute webinar with Q&A
Featured Webinar
Watch our webinar with Sanjay Vepa, MD, a specialist in pediatric and fetal cardiology, and Rupin Dhamankar, MS, CGC. Hear about:
- Two families impacted by 22q, and the profound impact prenatal detection can have on outcomes
- Best practices, guidelines, and the importance of early 22q screening
- How high detection 22q screening is possible with Panorama™ NIPT.
about noninvasive prenatal testing (NIPT) with Panorama™
about noninvasive prenatal testing (NIPT) with Panorama™
Affected pregnancies could be missed by lower detection 22q tests
Panorama™ detects 22q with the highest published clinical sensitivity from the largest, prospective NIPT study ever performed.1

There is currently no newborn screening for 22q
Nearly 50% of cases with 22q do not have any ultrasound findings10,11

Importantly, prenatal suspicion of 22q11.2 deletion syndrome allows for evaluation, confirmatory testing, and delivery at high-level healthcare facilities where neonates have access to potentially life-saving interventions including cardiac surgery, as well as treatment for other key features such as low calcium, immune deficiency, feeding, swallowing and breathing issues, all with the goal of optimizing long-term outcome while obviating the protracted diagnostic odyssey frequently traversed by families.” 12-14
Lauren
Patient
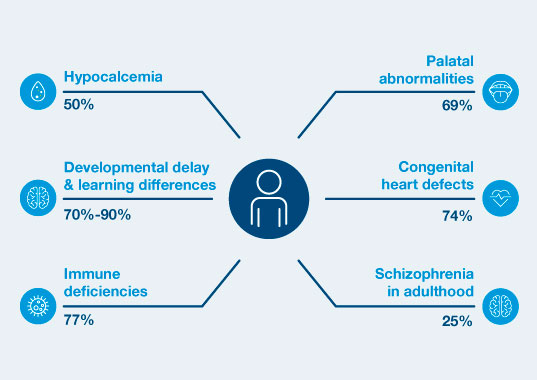
22q11.2 DS is the most common cause of syndromic palatal anomalies and most commonly known genetic cause of schizophrenia
In a published secondary analysis from SMART, >50% of patients at high risk for 22q by cfDNA screening were discharged without genetic confirmation.16 Early intervention can reduce the severity of these conditions associated with 22q11.2 deletion syndrome12-14, 17, 18
Prenatal diagnosis improves access to personalized care

22q11.2 DS is common
Up to 1:2,000 pregnancies may be affected by 22q11.2 deletion syndrome. There is currently no newborn screening for this condition.4 Maternal age is not a risk factor for having a pregnancy affected by 22q. Detection with ultrasound is limited, and nearly 50% of cases with 22q do not have any ultrasound findings.10,11
Panorama™ offers non-invasive screening which can lead to early detection.

Early identification matters
Patients visit an average of 7 different clinical specialties before they receive a molecular diagnosis of their condition, and the median time to diagnosis is 4.7 years when not detected prenatally.11 Patients with palatal and cardiac anomalies have a shorter average time to diagnosis.11
One patient’s journey to a diagnosis.

Early diagnosis improves outcomes
Families with a prenatal diagnosis can access interventions sooner that can help improve outcomes, and may have the option to deliver at a tertiary center. Families can have calcium-levels monitored at birth, delay live-vaccine administration or have a palatal evaluation for potential feeding and breathing issues.19,20
ACMG suggests “screening for 22q11.2 deletion syndrome be offered to all patients”3, 21
ACMG highlights the SMART study as the sole clinical study in support of their conditional recommendation (defined as a recommendation based on a moderate certainty of evidence).
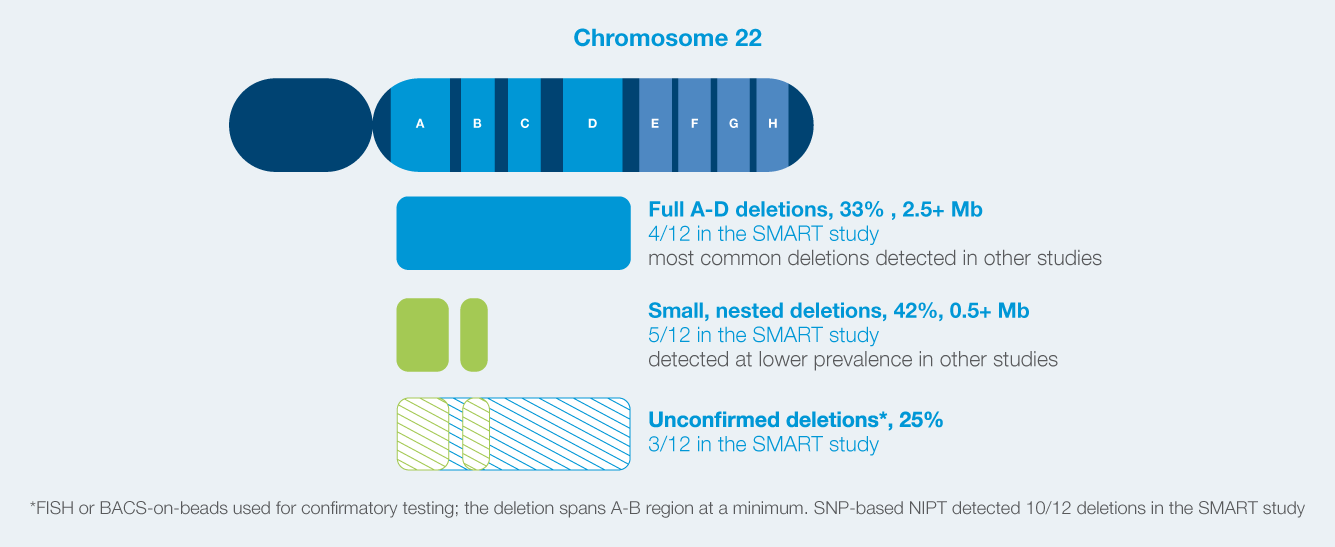
SNP-based NIPT is validated to identify deletions of all sizes
Smaller deletions can have the same degree of severity as full deletions and require similar intervention.22
Is Panorama™ right for you?
We’re here to help you find out
References
1Dar et al. Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am J Obstet Gynecol. 2022 Jul;227(1):79.e1-79.e11.
2Dar et al. Am J Obstet Gynecol. 2022 Aug;227(2):259.e1-259.e14.
3Gregg AR et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016 Oct;18(10):1056-65.
4Blagojevic et al. CMAJ Open. 2021 Aug 17;9(3):E802-E809.
5Hamosh et al. J Pediatr. 1998;132(2):255-259.
6O’Sullivan, Freedman. Lancet. 2009;373(9678):1891-1904.
7Prior et al. Spinal muscular atrophy. In: Adam et al, eds. GeneReviews. February 24, 2000.
8Snijders et al. Ultrasound Obstet Gynecol. 1999;13(3):167-170.
9Snijders RJ, et al. Fetal Diagn Ther. 1995 Nov-Dec; 10(6):356-67.
10Dinonno et al. Performance of SNP-based Cell-free DNA Prenatal Screening for 22q11.2 Deletion Syndrome in a Commercial Population, Society for Maternal-Fetal Medicine Pregnancy Meeting, February 2024, National Harbor, MD.
11Palmer et al. Am J Med Genet. 2018.
12Cheung et al. Genet Med. 2014 Jan; 16(1):40-4.
13Grand et al. Am J Med Genet A. 2018 Oct; 176(10):2167-71.
14McDonald-McGinn et al. 22q11.2 Deletion Syndrome. In: Adam et al., editors. GeneReviews. University of Washington,1993.
15McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015 Nov 19;1:15071.
16Martin K, Norton ME, MacPherson C, et al. Impact of high-risk prenatal screening results for 22q11.2 deletion syndrome on obstetric and neonatal management: Secondary analysis from the SMART study. Prenat Diagn. 2023;43(13):1574-1580. doi:10.1002/pd.6483.
17Morrow et al. Am J Med Genet A. 2018 176(10): 2070-2081.
18Bassett et al. J Pediatr. 2011 Aug; 159(2):332-9.e1.
19Ron HA et al. Genes (Basel). 2022 Dec 24;14(1):62.
20Freud et al. Am J Obstet Gynecol. 2023 Sep 16:S0002-9378(23)00611-7. doi: 10.1016/j.ajog.2023.09.005. Epub ahead of print.
21Dungan et al. Genet Med. 2023: 25 (2) 1098-3600.
22Dar P, Norton ME, Am J Obstet Gynecol. 2022 doi: https://doi.org/10.1016/j.ajog.2022.01.036.