Innovations in women’s health genetic testing
From prenatal genetic testing to informing cancer risk and recurrence, Natera continues to innovate its technology for broader applications that enable better women’s health outcomes.
Innovating at the intersection of oncology and prenatal genetics
Amber Shamburger, MD shares an example of how oncology and prenatal genetics intersect and one reason it’s important to be aware of what genetic testing offers in both fields.
Knowing that cancer diagnoses during pregnancy and within the first year post delivery are on the rise1,2,3 makes this an important discussion for those planning pregnancy or who are pregnant.4
Trusted expertise adapted to detect tumor DNA
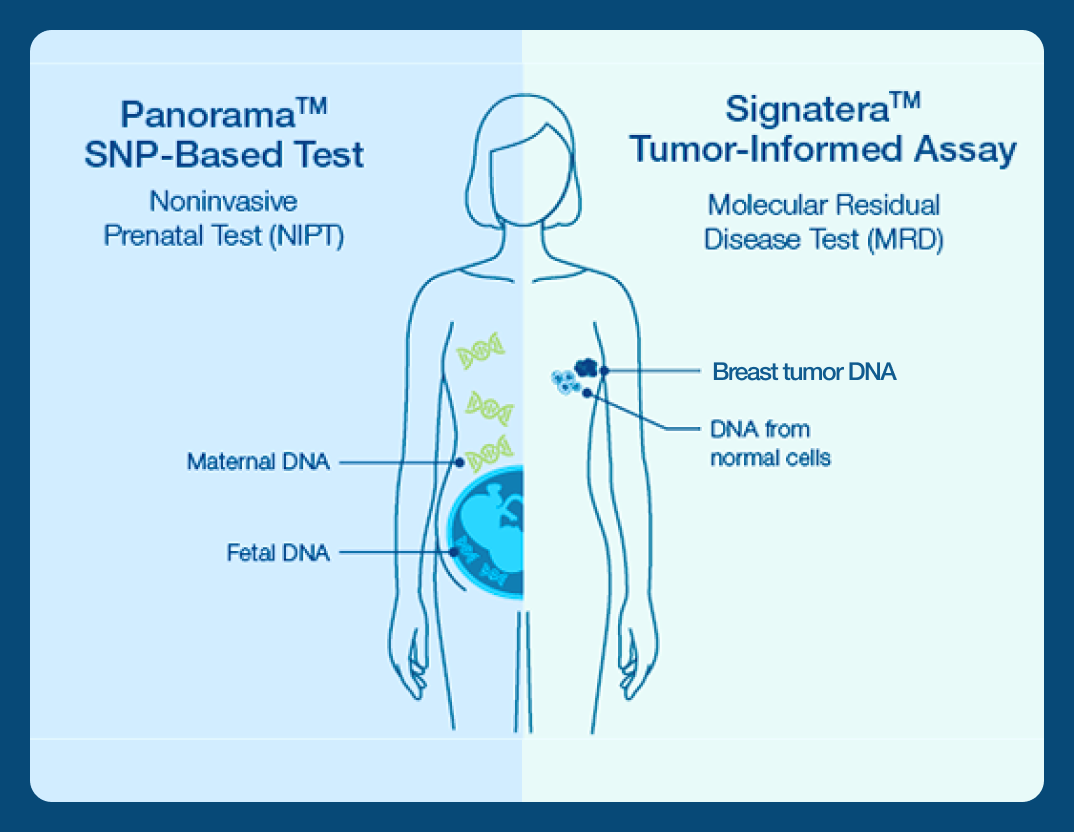
Pioneering NIPT technology now applied to cancer recurrence detection and monitoring
Natera introduced its pioneering single nucleotide polymorphism-based (SNP-based) NIPT 10+ years ago, which can distinguish maternal from fetal cell-free DNA (cfDNA). Now, this technology has been adapted to detect circulating tumor DNA (ctDNA) in those living with cancer.
Natera’s focus on rigorous clinical validation in prenatal testing continues with cancer genomics and precision medicine and is applied to each test in its portfolio. Natera is and will remain committed to extensively validating our offerings and publishing the data for scientific record.
Detecting cancer recurrence earlier
Signatera™ is the first tumor-informed molecular residual disease (MRD) test for individualized cancer care. It assesses the presence or absence of circulating tumor DNA (ctDNA) cells.
It’s used to detect cancer recurrence (or relapse) earlier, at the molecular level, than standard of care tools and for treatment response monitoring to indicate whether a therapeutic intervention is working or cancer is coming back.
See how Natera’s ctDNA test Signatera™ works
10.5 months earlier
median lead time in breast cancer recurrence detection over standard imaging52x the sensitivity
in detecting ovarian cancer relapse compared to CA-125, the most commonly used biomarker for ovarian cancer6,710 month lead time
on average, ahead of imaging as shown in a validation study in ovarian cancer6Recurrence monitoring and treatment response
Danielle’s Story
Danielle’s molecular breast cancer recurrence was detected by Signatera™ so that she and her doctor could act quickly. Hear her story and how recurrence monitoring is helping her live her life.
Angela’s Story
Angela faced many questions throughout her journey with breast cancer. Listen to how she was proactive in asking her doctor about Signatera™ to help monitor for recurrence and assess treatment response.
Testing to inform both cancer management and cancer risk
After diagnosis

Empower™ provides insight into why cancer developed and what treatment and surveillance options are available, as well as information for family members about their risk and testing options. Signatera™ helps identify relapse earlier than standard of care tools and detects response to neoadjuvant therapy and recurrence after adjuvant therapy, and monitors treatment response.
Without a diagnosis

Empower™ hereditary cancer test screens for genes associated with increased risk for common hereditary cancers for insight into the risk of developing 12+ specific cancers and why certain cancers may be common within their families. This can help inform whether adjusting a screening approach, utilizing preventive treatments or procedures, or lifestyle changes could be helpful.
Investing in innovations in personalized genetic testing
R&D INVESTMENTS
400 MILLION
PEER-REVIEWED PUBLICATIONS
250+
PATIENT SAMPLES
~12 MILLION
Learn more about Natera’s latest innovations
1Botha et al. FIGO Cancer Report 2018: Cancer in Pregnancy. Int J Gynecol Obstet. DOI: 10.1002/ijgo.12621.
2Maggen et al. Pregnancy and Cancer. The INCIP Project. Current Oncology Reports. 2020; 22:17.
3Matsuo et al. Assessment of severe maternal morbidity and mortality in pregnancies complicated by cancer in the US. JAMA Oncol. 2022;8(8):1213-1216. doi:10.1001/jamaoncol.2022.1795.
4Rink et al. Incidental detection of maternal malignancy by fetal cell-free DNA screening. Obstet Gynecol 140.1 (2022): 121-131.
5Shaw et al. Serial postoperative ctDNA monitoring for early detection of breast cancer recurrence. Poster presented at: ASCO; June 3-7, 2022; Chicago, IL.
6Hou et al. Circulating tumor DNA monitoring for early recurrence detection in epithelial ovarian cancer.Gynecol Oncol. 2022; 167:334-341.
7Chapman et al. Poster presented at 2021 AACR Annual Meeting.