Immunotherapy Awareness Month: Breakthroughs in Personalized Treatment for Advanced and Metastatic Cancer
Cancer immunotherapy has transformed the treatment of certain cancers and helped extend and save the lives of many people.
To support Cancer Immunotherapy Awareness Month, we’ll take a comprehensive look at this innovative treatment category. We will cover how immunotherapy works, the types of immunotherapy, advances in monitoring how people respond, and how you can join the cause to support immunotherapy awareness and research.
What is immunotherapy?
Cancer immunotherapy, also known as immuno-oncology (IO), is a type of treatment that uses the power of your immune system to better target and eliminate cancer cells. Different types of immunotherapy can be used to boost immune cells to help kill cancer cells, educate your immune system to spot and attack specific cancer cells, or make your immune response more effective.1
The main forms of immunotherapy used today include:
-
Monoclonal antibodies
-
Immune system modulators
-
T-cell transfer therapy
-
Cancer vaccines
-
Immune checkpoint inhibitors (ICIs)2
While immunotherapy can work well on its own, it can also be given in combination with surgery, chemotherapy, radiation, or targeted therapies to improve its effectiveness.
In recent years, immunotherapies have been approved in the United States and elsewhere to treat various types of cancer, including certain advanced, metastatic cancers that may have spread from where they first started to nearby tissue, lymph nodes, or other parts of the body. Some immunotherapies are also available through clinical trials, which are carefully controlled and monitored studies involving people with cancer.
Why use the immune system to treat cancer?
The immune system helps your body fight infections and diseases. It is made up of white blood cells (immune cells) and organs and tissues of the lymph system. While the immune system has evolved to detect and destroy abnormal cells, cancer has found ways of hiding from and switching off these immune responses. For example, cancer cells may:
-
Have genetic changes that make them harder to recognize as abnormal
-
Have proteins on their surface that interact with and turn off immune cells
-
Change the normal cells around the tumor so they interfere with how the immune system responds to the cancer cells
By either stimulating the immune system or reinforcing it, immunotherapy can help your body better fight against cancer.2
What are immune checkpoint inhibitors?
Immune checkpoint inhibitors (ICIs) treat advanced and metastatic cancers by taking advantage of how your immune system prevents attacks against normal cells. This mechanism involves immune checkpoints—the main “brakes” of the immune system that stop it from activating against normal cells. Some cancer cells can manipulate these checkpoints in order to disable immune responses and protect themselves from being destroyed.
ICIs—considered a new standard of care across many cancer indications—block immune checkpoints and help your immune system eliminate cancer cells by not only amplifying existing immune responses, but also unleashing new ones.
ICIs find and block specific immune checkpoint receptors, including:
-
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) on immune cells
-
Programmed cell death protein-1 (PD-1) on immune cells
-
Programmed cell death protein-ligand 1 (PD-L1) on normal and cancer cells
For example, when PD-1 on immune cells called T cells attaches to PD-L1 on cancer cells, it tells the T cells to leave the cancer cells alone. Blocking this PD-1/PD-L1 pathway can help restore cancer-fighting T cell activity. Similarly, blocking the CTLA-4 pathway can help T cells proliferate and become more diverse.3,4
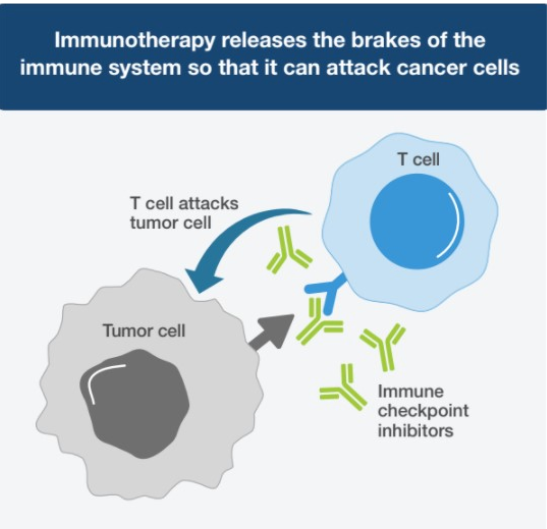
The first checkpoint inhibitor for the treatment of cancer—the CTLA-4-blocking ipilimumab (Yervoy)® for melanoma—was approved by the FDA in 2011. Today, nine ICIs are approved to treat more than a dozen different types of cancer.3
These approvals cover treatments for cancers that are advanced or resistant to other forms of therapy, as well as first-line treatments for several types of metastatic cancer. Many other ICIs are currently being evaluated in a variety of cancer types in clinical trials.
Why is monitoring ICI treatment response important?
Although ICIs are a relatively new and revolutionary cancer treatment option, they do not help all people with cancer:
-
About 43.5% of people with cancer are eligible for ICI treatment5
-
Less than 20% of eligible people will respond to or benefit from ICIs over the long term5
ICIs, like other cancer treatments, can cause side effects. Common side effects include fatigue, nausea, diarrhea, decreased appetite, cough, rash, and pain. More serious and rare side effects include reactions to the infusions and autoimmune responses that can cause problems in your organs and other parts of your body.4
Monitoring how you are responding to ICI treatment can help your doctor understand if the benefits of treatment outweigh the potential side effects. This information can help them better personalize your treatment plan. While imaging, biomarkers, and other lab tests are commonly used to monitor treatment response, they may not always provide a clear picture of whether ICIs are effective, or whether an alternative treatment strategy should be considered to avoid unnecessary exposure to the side effects and costs of ICI treatment.
How can SignateraTM help monitor ICI treatment response?
Signatera is a personalized cancer monitoring test that can help determine whether you are responding to ICIs earlier than traditional tests. Signatera assesses your levels of circulating tumor DNA (ctDNA)—small fragments of tumor DNA circulating in your bloodstream—to help you and your doctor track your response over time.
The INSPIRE trial showed Signatera's ability to identify people who did and did not respond to ICIs across 25 different cancer types. Signatera was able to predict immunotherapy response as early as 6 weeks into treatment.6 When used alongside standard monitoring tests, Signatera can help you and your doctor get an early understanding of your response to ICIs and whether to continue with or adjust your treatment strategy.
A new clinical trial is further examining the impact of incorporating Signatera testing in immunotherapy management for people with advanced or metastatic solid tumors. Learn more about the BESPOKE IO trial, which is currently enrolling people living with colorectal cancer, non-small cell lung cancer, and melanoma.
How can you learn more and join the cause?
Many online resources are available to help you learn about immunotherapy’s life-changing impact. These include Natera’s partners: Society for Immunotherapy of Cancer (SITC), European Society for Medical Oncology (IO), and AIM with Immunotherapy, which also offers plenty of resources customized by the region of the world. You can find additional comprehensive immunotherapy resources below.
To help raise immunotherapy awareness, you can participate in fundraising efforts and connect with the immunotherapy community during the month of June and beyond. You can also check out the 10th Annual Cancer Immunotherapy Month™ hosted by the Cancer Research Institute.
Additional Resources
References
1Cancer Research Institute. What is Cancer Immunotherapy? Accessed May 23, 2022. https://www.cancerresearch.org/immunotherapy/what-is-immunotherapy
2National Cancer Institute. Immunotherapy to Treat Cancer. Accessed May 23, 2022. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
3Cancer Research Institute. Immunomodulators: Checkpoint Inhibitors, Cytokines, Agonists, and Adjuvants. Accessed May 23, 2022.
https://www.cancerresearch.org/immunotherapy/treatment-types/immunomodulators-checkpoint-inhibitors
4American Cancer Society. Immune Checkpoint Inhibitors and Their Side Effects. Accessed May 23, 2022. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html
5Haslam A, et al. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Network Open. 2019;2(5):e192535-e192535. https://doi.org/10.1001/jamanetworkopen.2019.2535
6Bratman SV, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873-881. https://doi.org/10.1038/s43018-020-0096-5
