Finding hope with Signatera™
When Steven was diagnosed with metastatic melanoma, he started to lose hope. After his oncologist ordered circulating tumor DNA (ctDNA) testing with Signatera™, Steven found the peace of mind he needed to rediscover his purpose and joy. His tumor tissue was sequenced for its unique genomic variants, and now his oncologist uses serial blood draws to confirm that Steven remains cancer-free during immunotherapy. Watch Steven’s story now to learn more.

Steven
Immunotherapy patient
Learn how Signatera™ works
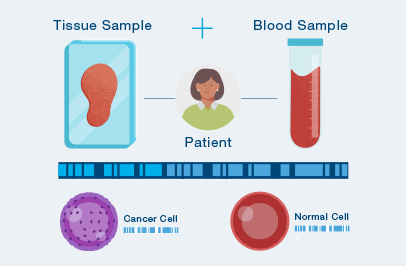
A one-time analysis of both blood and tissue determines your unique set of tumor mutations.

The test is custom-built and personalized for you.
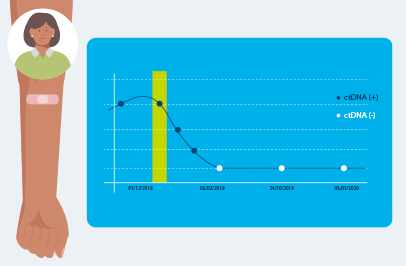
Signatera™ detects the presence or absence of cancer each time it is ordered as part of your routine follow-up blood tests.
Learn how Signatera™ detects your cancer
Understand your Signatera™ test results
Your test results will either be positive or negative for the presence of ctDNA in your blood. Your doctor will receive the test report and discuss your results and answer questions.
IMPORTANT: Negative results may change over time. A negative Signatera™ result doesn’t guarantee that tumor DNA is not in your blood, or that it will never be detected in the future. This is why ongoing monitoring with the Signatera™ test over the course of your cancer care, as directed by your doctor, is recommended for early detection of residual disease.
Access Personalized Testing
Medicare Coverage
- Signatera™ is covered by Medicare for monitoring immunotherapy response in patients with any solid tumor.
- We welcome all insurance plans. Please refer to our list of in-network plans that we participate with, or call your insurance provider.
- We offer financial assistance programs for those that qualify.
More Information
- Find answers to your questions about eligibility, results, ordering, and more
- Don’t see your question? Contact us here.