Inform Decisions for High Risk Patients
Risk-Stratify Patients
Identify patients with residual disease after surgery who may benefit from adjuvant treatment for high risk individuals
5.6x
higher risk of distant metastasis in patients with molecular residual disease (MRD) after surgery1
Detect Recurrence Earlier
Use Signatera™ serially to monitor and identify breast cancer patients at high risk of recurrence2
52x
higher risk of relapse in ctDNA positive versus ctDNA negative patients
Assess Treatment Response
Track changes in ctDNA levels to evaluate treatment response3
6 weeks
to understand response to immunotherapy treatment3

WEBINAR
Tiny Traces, Big Impact:
Changing the Paradigm in Breast Cancer Management with MRD Testing
60 minute webinar
Featured webinar
Tiny Traces, Big Impact: Changing the Paradigm in Breast Cancer Management with MRD Testing
In this AI in Precision Oncology webinar, Arielle Medford, MD, a breast cancer expert, will discuss the prognostic and predictive power of MRD testing. Key objectives of this webinar
- How MRD testing is being integrated as a reliable decision-making tool to guide adjuvant therapy decisions with the goal of right-sizing treatment
- The utility of serial MRD testing for surveillance and early identification of molecular relapse—often months before clinical progression
- Clinical evidence and ongoing trials supporting MRD testing in clinical practice
- How MRD-guided therapy may be used to improve DFS, enable earlier intervention, and personalize patient management based on molecular risk
Get Started
Detect Recurrence Earlier
- Up to 30% of breast cancer patients with no evidence of disease after curative intent treatment will eventually relapse and succumb to their disease4 — suggesting that existing tools are inadequate at identifying recurrence before the patient becomes symptomatic
- Approximately 10-15% of patients experience reduced performance status upon cancer recurrenceand treatment options are limited5
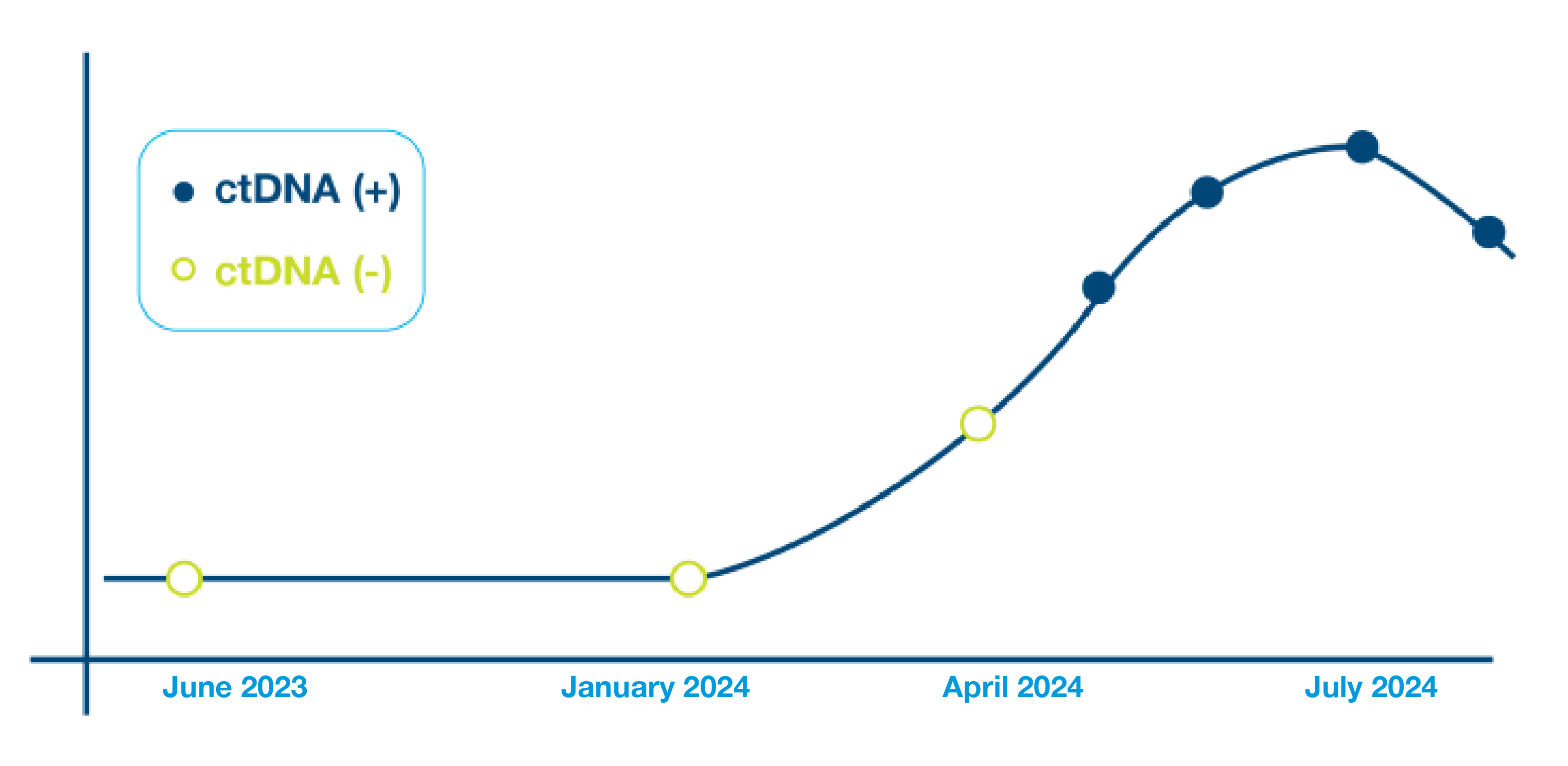
- Longitudinal testing with Signatera™ has been shown to identify patients at high risk of recurrence with 88% sensitivity and a median of 10.5 months (range 0-38 months) ahead of imaging2, potentially enabling meaningful and timey intervention
Inform Questions Across Breast Cancer Treatment
Explore the Peer-Reviewed Data in Breast Cancer
Neoadjuvant Response Monitoring and Risk Stratification
I-SPY 2 Study Schema
I-SPY 2 Studies: Can ctDNA Refine pCR as a Surrogate Endpoint for Survival?
For patients treated with neoadjuvant therapy, achieving a pathologic complete response (pCR) generally denotes a favorable prognosis. However, how can providers risk-stratify patients who have residual disease?
I-SPY 2 ctDNA Objectives
- Evaluate ctDNA as a biomarker for monitoring response to neoadjuvant therapy6,7
- Assess prognostic value of ctDNA in stratifying patients for risk of early metastatic recurrence6,7
The I-SPY 2 Platform Master Protocol
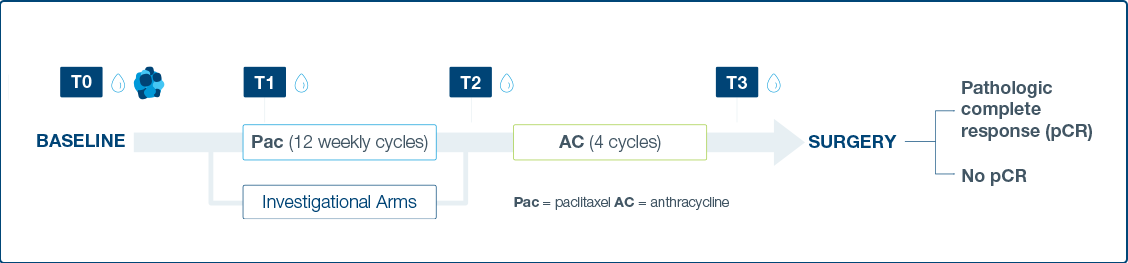
- Response to neoadjuvant chemotherapy (NAC) and investigational treatments assessed through ctDNA dynamics at four time points
- ctDNA derived from plasma was assessed at four time points: pretreatment (T0), 3 weeks after paclitaxel/study drug initiation (T1), between
paclitaxel/study drug and anthracycline regimens (T2), and prior to surgery (T3).
I-SPY 2 Results
pCR and ctDNA status Can Help Risk-Stratify Patients6,7
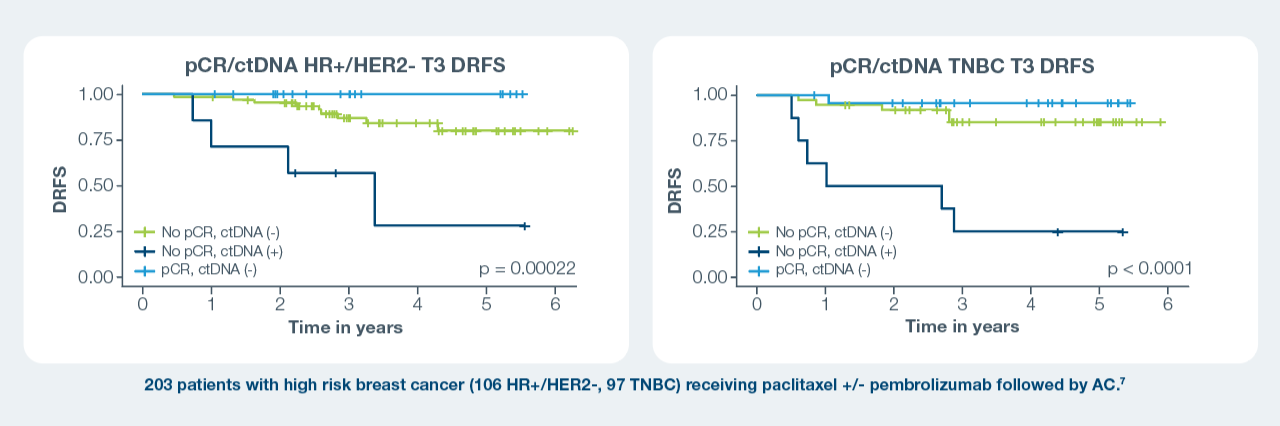
At T3 across subtypes:
- Patients who achieved a pCR and cleared their ctDNA had the best survival outcomes; those who did not achieve a pCR and were ctDNA positive
had the worst outcomes.1–3 - Regardless of pCR status, persistent ctDNA positivity by T3 was associated with shorter DRFS compared to ctDNA negativity.1–3
Adjuvant MRD Assessment
Risk stratify patients after surgery to help identify those with residual disease who may benefit from intensified or extended adjuvant treatment
The EBLIS Study:
ctDNA status in conjunction with existing tools after surgery and adjuvant chemotherapy identified high risk patients with poor recurrence-free survival8 who may benefit from adjuvant treatment for high risk individuals
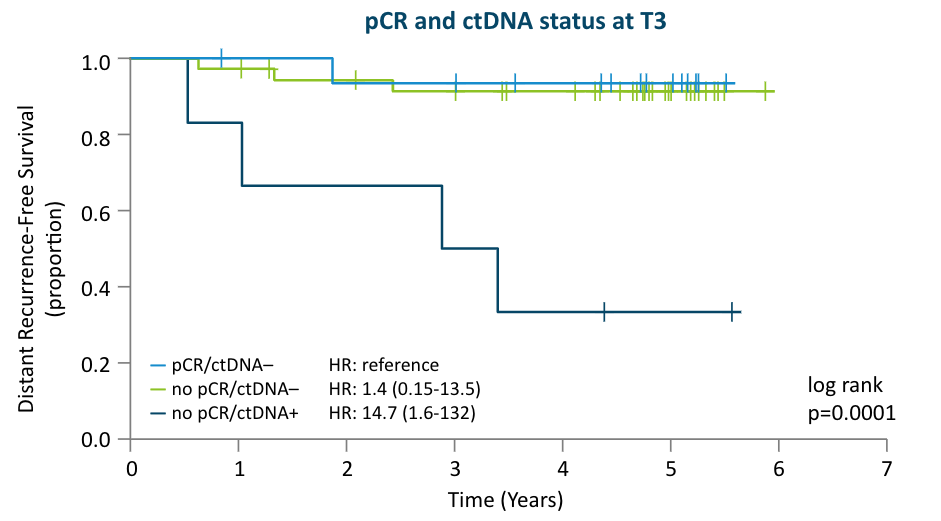
The I-SPY 2 Study:
Prior to surgery, the I-SPY 2 study showed that lack of ctDNA clearance regardless of pCR status identified patients with poor response to neoadjuvant chemotherapy and a high risk of recurrence6, which can help inform adjuvant treatment decisions
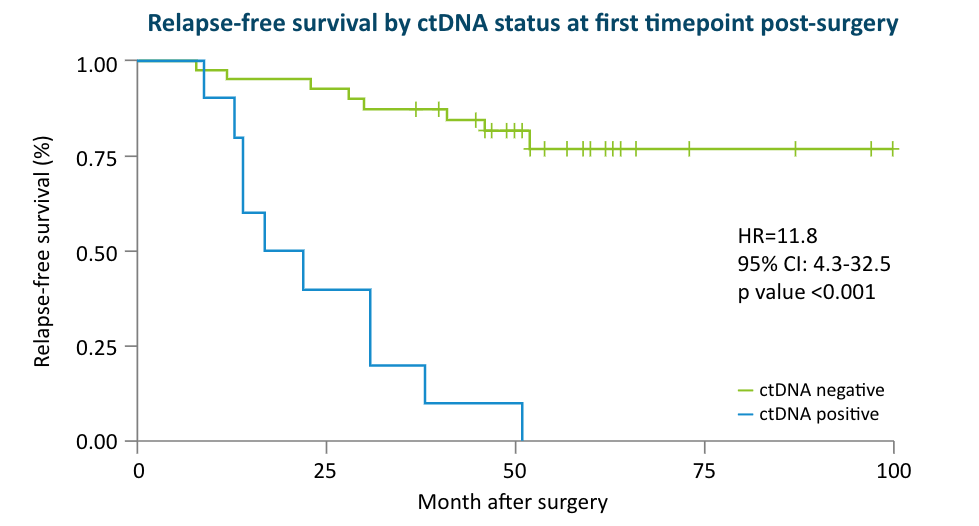
The Early Breast Cancer Study:
48 early stage breast cancer patients receiving primary surgery included in analysis (40 also received chemotherapy), with 144 total plasma samples; median follow up time 5 years (0.5-6)1

- 38% of patients who were ctDNA positive after surgery relapsed, while the remaining possibly benefited from adjuvant therapy.1
- Detection of ctDNA after surgery was associated with worse outcomes compared to no ctDNA detection (DMFS HR: 5.6; 95% CI: 1.1-29.3; P=0.04)1
- These data suggest that although MRD positivity post-surgery is prognostic of patient outcomes, it may be modifiable with adjuvant therapy.1
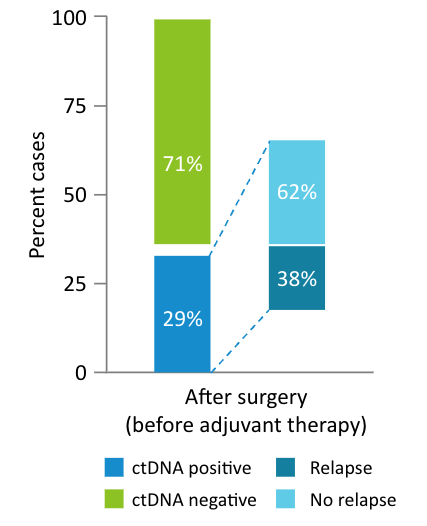
Surveillance Recurrence Monitoring
The EBLIS Study: Is there a more sensitive technology that can detect recurrence earlier?
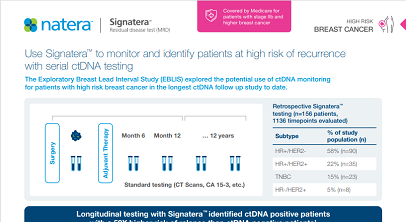
The Exploratory Breast Lead Interval Study (EBLIS) explored the potential use of ctDNA monitoring in patients with high risk breast cancer in the longest ctDNA follow up study to date.2
EBLIS Protocol
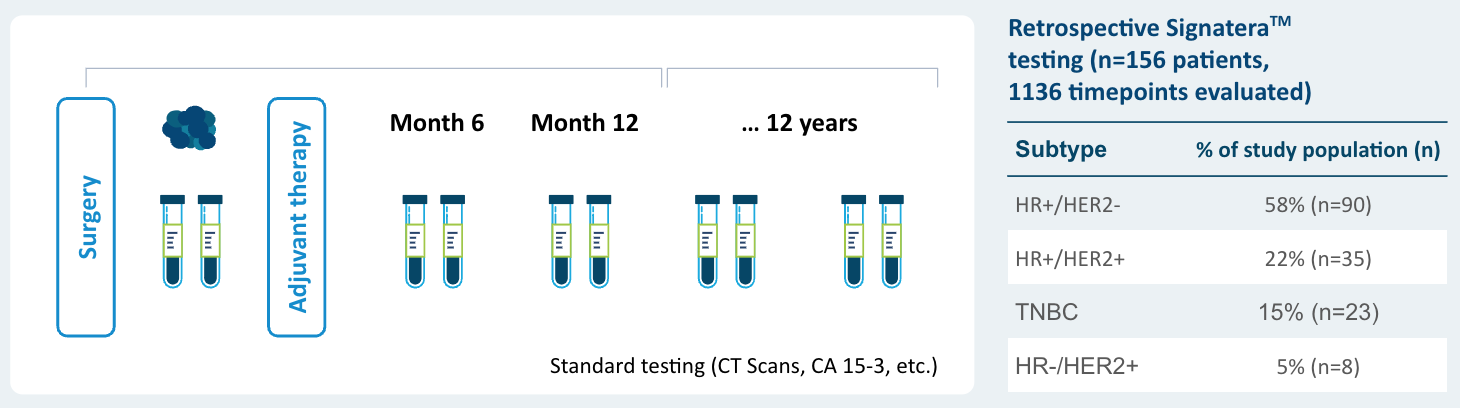
EBLIS Results
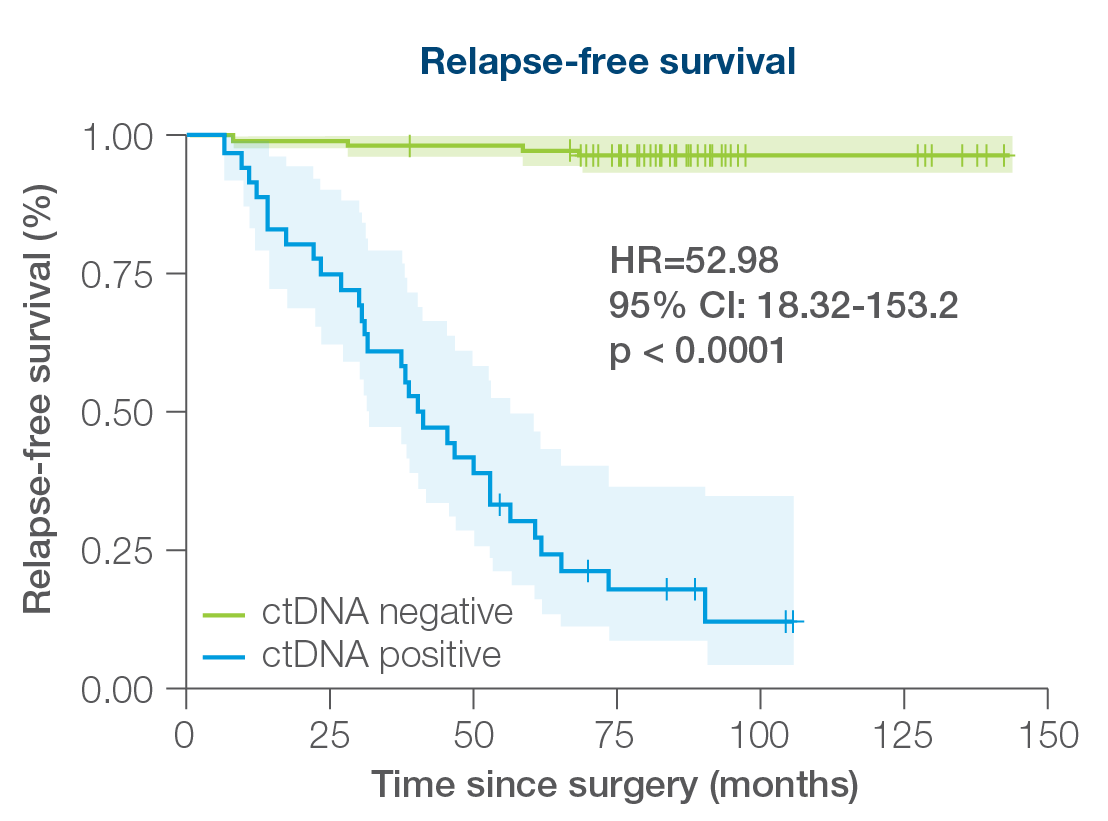
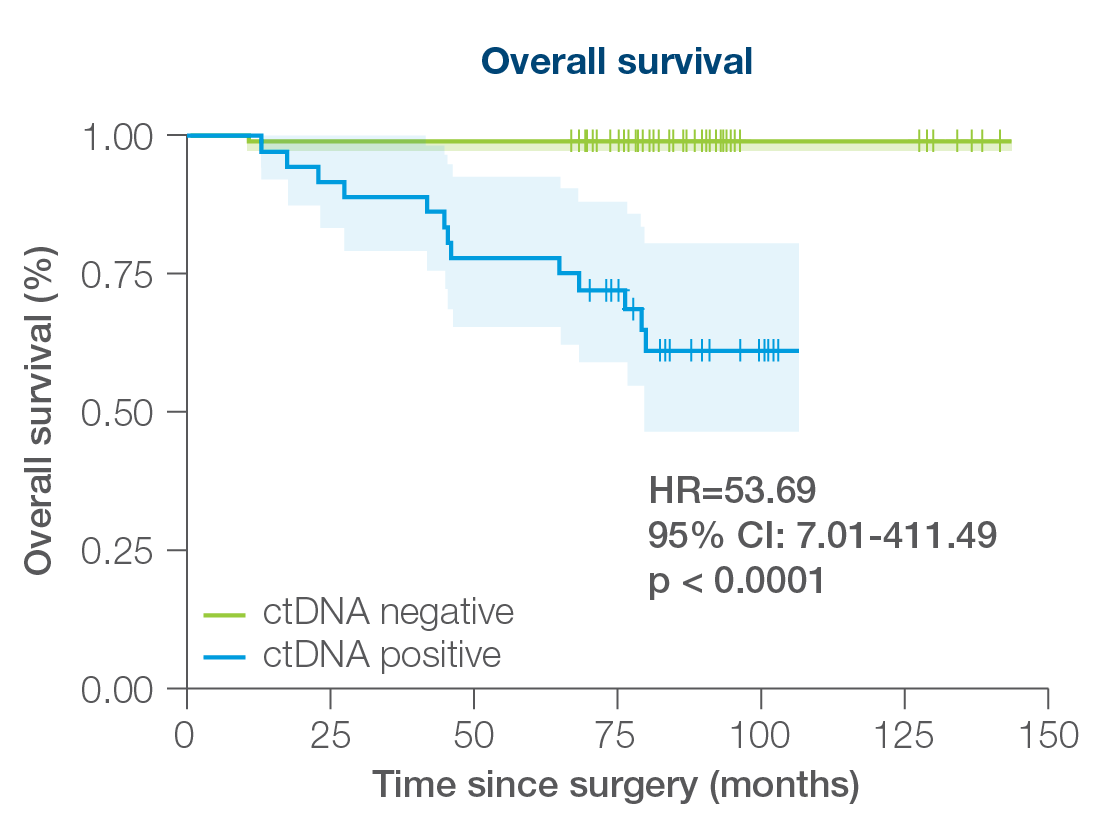
Signatera™ can be used serially to monitor and identify breast cancer patients at high risk of recurrence
- 156 patients followed over a peroid of 12 years including all breast cancer subtypes
- 88% sensitivity: Signatera™ detected ctDNA in 30 out of 34 patients before clinical or radiologic relapse
- 10.5 months median lead time over radiographic recurrence (range 0-38 months)
Assess Immunotherapy Response
The INSPIRE Study: Can ctDNA be Validated as an Early Biomarker of ICI Treatment Response?
Despite dramatic improvements in cancer care using antibodies that block immune checkpoint proteins, less than 20% of eligible patients will derive sustained response or clinical benefit to immune checkpoint inhibitors (ICIs).7 How can ctDNA accurately identify those patients who are benefiting from ICI treatment?
INSPIRE Objectives
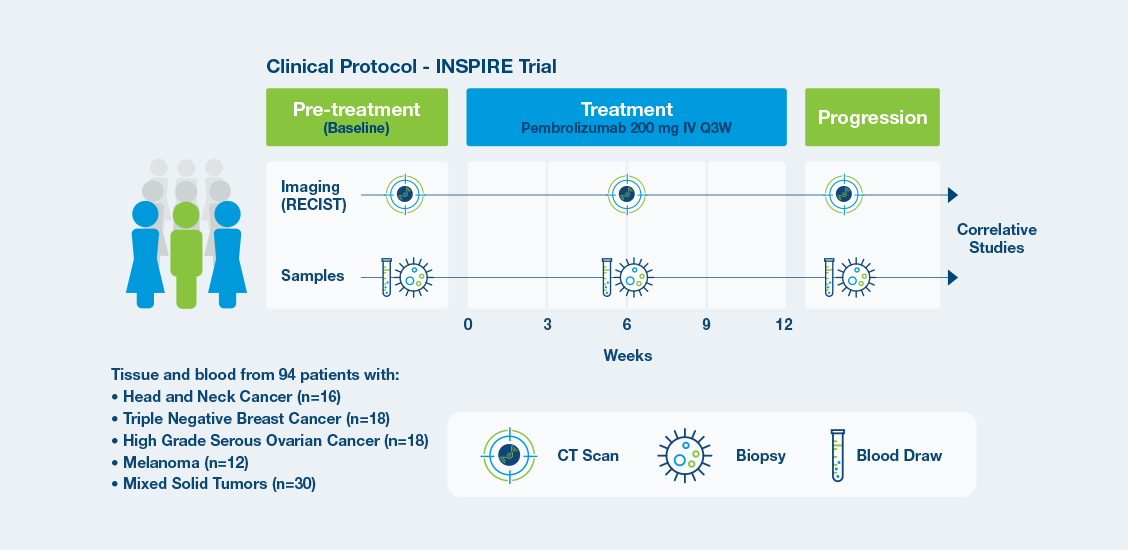
The prospective phase II INSPIRE trial addressed clinically relevant issues related to monitoring response to ICIs by assessing baseline ctDNA status and ctDNA dynamics.
INSPIRE Protocol
INSPIRE Results
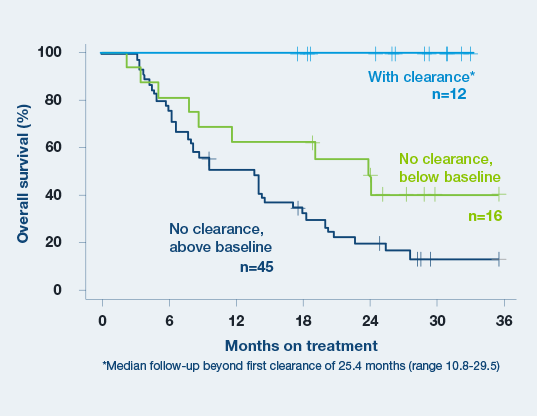
Signatera™ predicts immunotherapy benefit as early as week 6.3
Baseline ctDNA concentration and ctDNA dynamics during treatment correlated with progression-free survival, overall survival, clinical response, and clinical benefit.3
- A decrease in ctDNA levels from baseline to the beginning of cycle 3 is a strong predictor of increased OS and PFS.3
- Clearance of ctDNA at any time point resulted in superior clinical outcomes. All 12 patients who cleared ctDNA for at least one on-treatment time point remained alive during follow-up period (median follow-up beyond first clearance of 25.4 months)3
Guiding the Breast Cancer Patient Journey
Angela’s Story
Like many patients, Angela has faced many questions throughout her journey with high-risk breast cancer – what’s the next step in my treatment? Is the cancer going to come back?
Listen to Angela’s story of how she and her care team used Signatera™ to help monitor for recurrence and therapy response, giving her the peace of mind to move forward with her life.
Covered by Medicare for multiple solid tumor indications
Learn More
Ready to try Signatera™ for your breast cancer patients?
References
1Cutts R, et al. Detection of ctDNA following surgery predicts relapse in breast cancer patients receiving primary surgery. SABCS; December 7-11, 2021.
2Shaw JA, et al. Serial postoperative ctDNA monitoring for early detection of breast cancer recurrence. ASCO; June 3-7, 2022.
3Bratman SV, et al. Nature Cancer. 2020;1(9):873-881.
4Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2005;365:1687-1717.
5O'Connor T, et al. Clin Adv Hematol Oncol. 2013;11(6):341-347.
6Magbanua MJM, et al. Ann Oncol. 2021; 32(2):229-239.
7Magbanua MJM, et al. Comparison of the predictive and prognostic significance of circulating tumor DNA in patients with high risk HER2-negative breast cancer receiving neoadjuvant chemotherapy. AACR; April 8-13, 2022.
8Coombes RC, et al. Clin Cancer Res. 2019 Jul 15;25(14):4255-4263.