The Basics of Prenatal Genetic Screening
Navigating pregnancy can be daunting. If you are pregnant or trying to conceive, you likely have many questions: What should I eat? How should I exercise? Which vitamins should I take?
You are also likely wondering about your baby’s health. Prenatal genetic screening can help you learn whether your baby is at risk for a genetic condition and whether further, more invasive, diagnostic testing may be necessary. Diagnostic testing confirms whether your baby will be born with a genetic condition. Having access to this information during pregnancy can enable families and their doctors to prepare to support a baby who could require an extra level of medical care. For example, some families decide to give birth at a specialized hospital, update their insurance coverage, and identify specialists early.
There are many different types of prenatal genetic screening. Understanding your options一and how to interpret your results一can help you choose the most appropriate screening for you.
What Is a Genetic Condition?
A genetic condition, sometimes called a genetic disorder, is a health condition caused by a change in a person’s DNA that can impact how their body functions. Changes can occur in genes (the DNA instructions for how cells function) or in chromosomes (the structures that hold our DNA). Due to random errors in the egg or the sperm, a baby can inherit a genetic condition from their parents or develop one at conception. Worldwide, about 6% of babies born each year have a condition that is genetic or partly genetic in origin.1
Some genetic conditions have serious effects on a baby’s health and development, while others have milder effects. Certain genetic conditions, such as Down syndrome, can have a wide range of possible health and developmental effects that can only be fully assessed after a baby is born.2
Understanding the Difference Between Screening and Diagnostic Tests
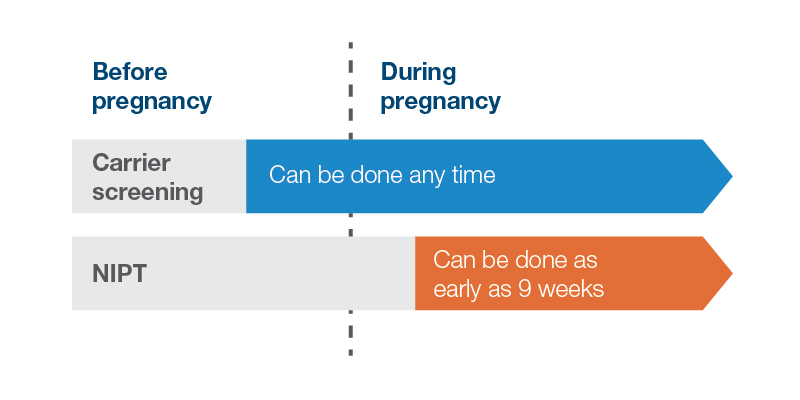
Prenatal genetic screening tests are performed before or during pregnancy to check your baby’s risk of being born with certain genetic conditions. You’ll only need a standard blood draw to receive a noninvasive screening test, and therefore there is no increased risk of miscarriage.
The most important thing to know about any prenatal genetic screen is that it is not designed to provide a diagnosis. Prenatal screening can only assess your potential risk of having a baby with a specific genetic condition一not determine with certainty whether the baby has that condition. For this reason, critical decisions should not be based on the outcome of a prenatal screen alone.
In contrast, diagnostic tests are used to tell you with as much certainty as possible if your baby has a particular genetic condition.3
The most common prenatal diagnostic tests are chorionic villus sampling (CVS) and amniocentesis. CVS and amniocentesis require a sample of tissue or fluid, which is extracted from the baby’s placenta or amniotic fluid, respectively. These tests are invasive, and therefore they carry a very small chance of miscarriage.4 CVS can only be performed during a relatively short window of time in pregnancy, while amniocentesis must be performed later in pregnancy than prenatal screening is available.
Your provider will offer diagnostic testing if your prenatal screening shows an elevated risk of your baby having a genetic condition. You can choose to undergo diagnostic testing if you want to know with certainty whether your baby has the genetic condition in question. If your diagnostic test confirms the result, you can work with your provider and/or genetic counselor to learn about the condition and plan for future medical care that your baby could need.
Prenatal genetic screening and diagnostic tests require a doctor’s order.5 Ultimately, you and your provider should discuss the pros and cons of the tests that are available to you.
If you choose to have prenatal genetic screening, there are 3 important types to consider:
- Carrier screening
- Noninvasive prenatal testing (NIPT)
- Single-gene noninvasive prenatal testing (single-gene NIPT)
What is Carrier Screening?
As the name suggests, carrier screening tests you and your partner to learn if you carry a gene with a change, or mutation, that can cause genetic conditions that could be passed onto your baby. Carrier screening differs from other prenatal screening in a few ways. Ideally, it is completed before pregnancy to enable you to have more time to make decisions about your reproductive options but can also be performed alongside other prenatal screens when you are already pregnant.6 Unlike most other prenatal tests, your carrier status will stay the same throughout your life. Your risk of passing a condition on will not change from pregnancy to pregnancy, unless you conceive with a different partner in the future.
Carrier screening can help you understand your baby’s risk of being born with a serious inherited genetic condition, such as cystic fibrosis, sickle cell anemia, or fragile X syndrome.
How Does Carrier Screening Work?
Humans usually have two copies of each gene, one copy from each biological parent. Being a carrier of a condition means that you have one healthy copy of the gene associated with that condition, and a second copy that is missing or mutated. Carriers are usually healthy and often do not have a family history of the condition they carry.
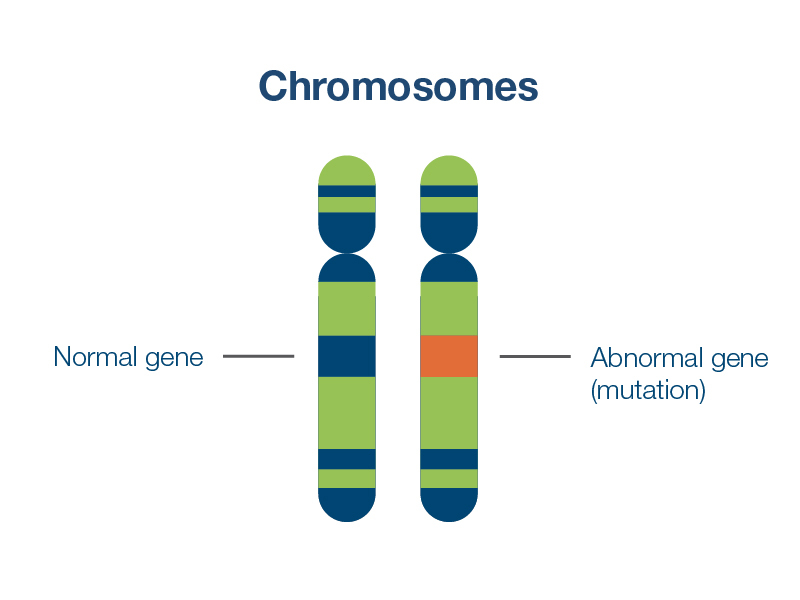
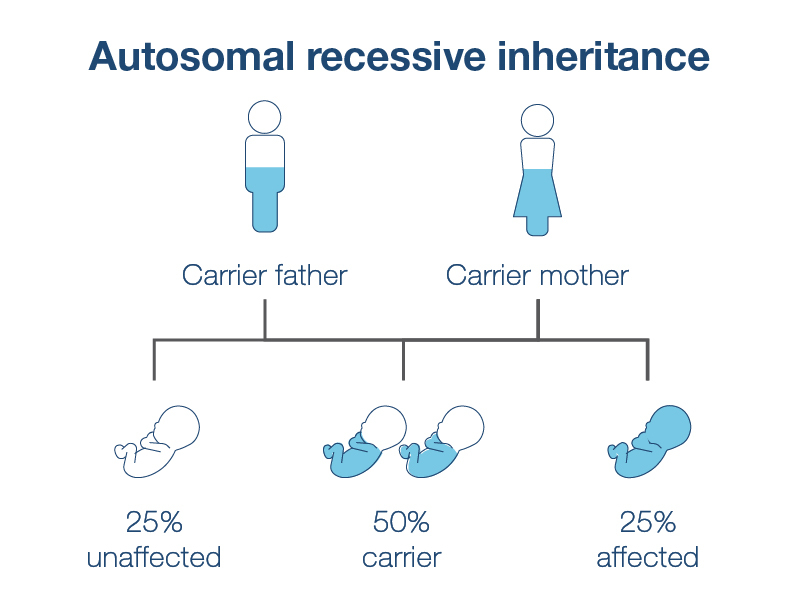
Carrier screening can detect autosomal recessive genetic conditions, such as cystic fibrosis, which require that a person inherits two mutated genes, one from each parent, to be impacted by the genetic condition. If you and your partner are both carriers for the same autosomal recessive genetic condition, your child has a 1 in 4 (25%) chance of inheriting that condition. For this reason, if one parent is found to carry an autosomal recessive genetic condition, your doctor could recommend that the other parent be tested . Partner testing can be done at the same time as your testing, or after your test results are completed.
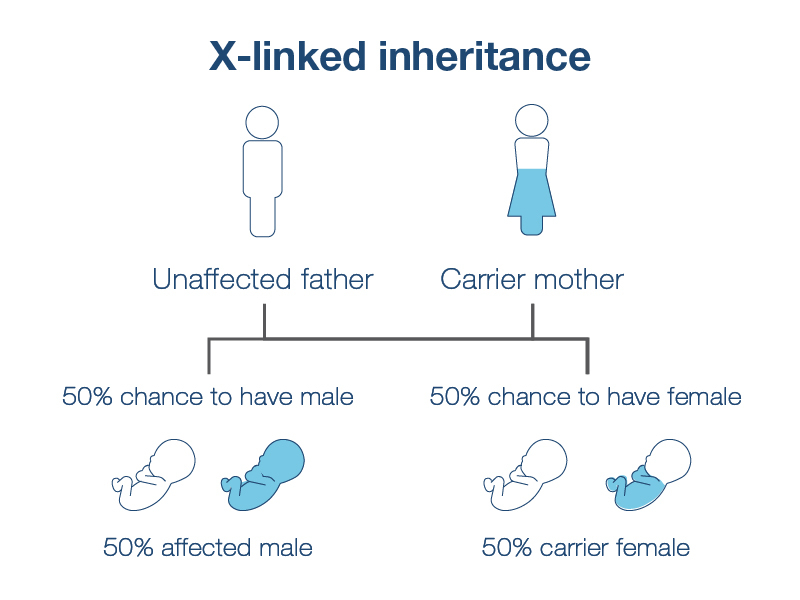
Carrier screening also determines your risk of having a baby with an X-linked condition. These are conditions caused by mutations in genes found on the X chromosome. If you carry an X-linked condition, there is a 1 in 4 chance of having an affected baby—1 in 2 if you know your baby is biologically male. This risk is not impacted by your partner's carrier status. Typically, males are not carriers of X-linked conditions and do not need to be screened for them.
Duchenne muscular dystrophy (DMD) is an example of an X-linked condition. It can cause progressive muscle weakness that usually begins in the lower limbs. DMD also causes heart and lung problems that worsen. It usually affects boys, although in rare cases it can affect girls. Symptoms can begin as early as age 2 or 3, and many children with DMD need to use a wheelchair around age 127. The average life expectancy for a person with DMD is about 27 years.8 Prenatal diagnosis allows for early treatment that can help improve quality of life for children with DMD.
Who Should Get Carrier Screening?
Everyone can benefit from carrier screening. Most people are carriers for at least one condition without knowing it. Since it takes two mutated or missing copies of the same gene for a condition to appear, there are usually no signs or symptoms of being a carrier and genes can stay in families for many generations without affecting anyone.9 For example, 88% of carriers of cystic fibrosis, spinal muscular atrophy, and fragile X syndrome have no known family history of the condition.10
The American College of Obstetrics and Gynecology (ACOG) supports offering carrier screening for at least cystic fibrosis, spinal muscular atrophy (SMA), and sickle cell disease to everyone who is pregnant or considering pregnancy. Carrier screening for other conditions, like fragile X syndrome and Tay-Sachs disease, should be offered if you have certain known risk factors, like a particular ethnic background or a personal or family history that suggests a higher risk of carrying the gene.
Ultimately, whether or not you choose carrier screening is up to you and your provider. If you choose carrier screening, the next step is to select which test to use and which conditions to screen for.
HorizonTM for Carrier Screening
Horizon is an advanced carrier screen that has been shown to detect more carriers than traditional screening.11 It uses cutting-edge next-generation DNA-sequencing technology to screen individuals for up to 200+ genetic conditions. Your provider or genetic counselor can help you determine exactly which conditions to screen for. Testing focus can depend on your family history and ethnic background, as some conditions are more common in certain ethnic groups. The standard Horizon panel screens for 14 conditions, including:
- Cystic fibrosis
- Spinal muscular atrophy
- Tay-Sachs disease
- Duchenne/Becker muscular dystrophy
- Hemoglobinopathies, like Sickle Cell Disease
On average, 1 in 9 people screened with Horizon are a carrier for one of these 14 conditions, and one in 634 babies is born with one of these conditions.11
Ask your provider about Horizon for carrier screening, or schedule a complimentary information session with a Natera genetic counselor.
Learning More About Your Baby With NIPT
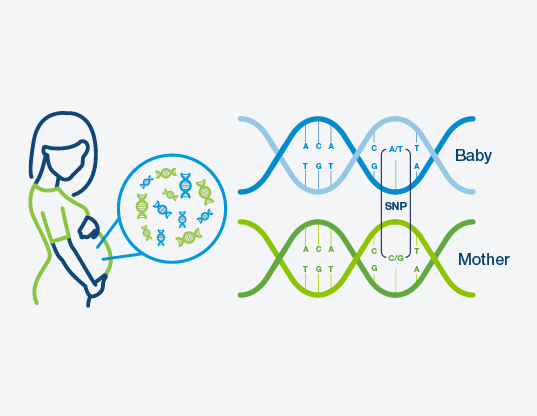
Once you are pregnant, it becomes possible to screen your growing baby for genetic conditions that could be unique to them and not covered by carrier screening. Noninvasive prenatal testing (NIPT)—also known as noninvasive prenatal screening (NIPS)—uses fragments of a baby’s DNA floating in your bloodstream, called cell-free DNA (cfDNA), to determine whether your baby is at high or low risk for specific genetic conditions. NIPT can also accurately determine your baby’s biological sex.12
In contrast to carrier screening, which looks at the genes of one or both parents, NIPT looks at your baby’s chromosomes. By analyzing your baby’s chromosomes, NIPT screens for some of the more common genetic conditions related to chromosomal differences. These conditions typically happen by chance and are not usually inherited from either parent. Most of these involve aneuploidies, which are caused by extra or missing chromosomes.
Some of the conditions screened for by NIPT are:
- Down syndrome (trisomy 21)
- Edwards syndrome (trisomy 18)
- Patau syndrome (trisomy 13)
- Conditions caused by changes in the sex chromosomes (e.g., Turner and Klinefelter syndromes)
Some NIPTs screen for microdeletions (small missing pieces of a chromosome) that are associated with certain genetic conditions, such as 22q11.2 deletion syndrome.
Since NIPT was introduced in 2011, an increasing number of pregnancies have been screened using NIPT. This increased use is because:
- It is convenient and safe to perform with a standard blood draw
- It offers higher accuracy than other prenatal blood tests
- It can be done earlier in pregnancy than other screening (as early as 9 weeks), providing more time to make decisions in the case of high-risk results
Like all prenatal genetic testing, NIPT occasionally can have false positive and false negative results. If NIPT results indicate a high risk, or “screen positive,” result for a genetic condition, you and your provider should consider diagnostic testing with CVS or amniocentesis to confirm or rule out the condition. Decisions about your pregnancy should not be made based only on the results of NIPT.
Who Should Get NIPT?
In 2020, ACOG began supporting providers offering NIPT to all pregnant people regardless of age or risk factors. NIPT is an excellent screening choice because it provides a noninvasive and highly accurate risk assessment for certain genetic conditions.
NIPT technologies that use SNP-based DNA analysis offer more accurate results for low risk pregnancies than previous methods, which were only recommended for high risk individuals.Your provider or genetic counselor can help you decide whether NIPT is right for you.
How Does NIPT Work?
NIPT can be performed as early as nine weeks of pregnancy. NIPT requires a blood sample, which contains fragments of DNA from the baby’s placenta. This DNA is called cell-free DNA (cfDNA) because it is not contained within a cell. NIPT uses cfDNA to determine your baby’s risk of having certain genetic conditions.
Personalized NIPT With Panorama™
Not all NIPTs are created equal. Evaluating the performance of an NIPT is critical when choosing the right one for you.
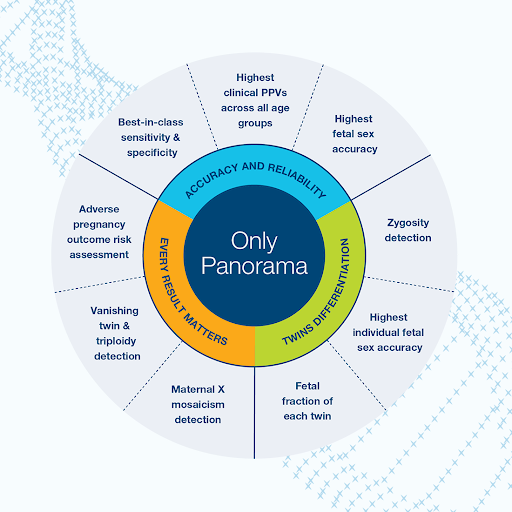
Over 3 million pregnancies have been screened with the Panorama NIPT. Panorama uses a unique kind of genetic analysis based on single nucleotide polymorphisms (SNPs)—the 1% of DNA that makes each of us different—along with proprietary artificial intelligence (AI) analysis. Unlike other NIPTs, Panorama’s SNP-based technique can distinguish between your DNA and the baby’s DNA. This technology allows Panorama to more accurately assess your baby’s risk of having a genetic condition.
Panorama detects more than 99% of pregnancies affected by trisomy 21 (Down syndrome). More than 9 out of 10 high risk Panorama results for trisomy 21 are confirmed to be truly affected—this is called the positive predictive value (PPV). Unlike some NIPTs, Panorama’s PPV for trisomy 21 does not decrease significantly for younger pregnant indidviduals.13-14
Panorama’s optional microdeletions panel demonstrates the highest sensitivity currently available for detecting 22q11.2 deletion syndrome, a leading cause of neurodevelopmental delay and congenital heart abnormalities.15 This genetic condition is relatively difficult to diagnose after birth because it can present in many different ways.16 Due to the broad range of symptoms, the average child with 22q11.2 deletion syndrome is not diagnosed until age 4 .17 Prenatal detection and early treatment can improve long-term health outcomes for these children through earlier treatment.
Panorama offers many and more unique features, including detection of:
- Vanishing twins (a twin that disappears in embryo)
- Triploidy (an extra full set of chromosomes)
- Identical (monozygotic) or non-identical (dizygotic) twins, which can help your provider plan appropriate care
Panorama also has the highest published accuracy for determining a baby’s biological sex. If you have a non-identical twin pregnancy, it can even determine the sex of each twin.
Panorama results are reported as “high risk” or “low risk,” rather than “positive” or “negative,” to clarify that it can only determine risk, not the presence or absence of a particular condition.
Panorama can be performed starting at nine weeks of pregnancy. Learn more here.
NIPT for Single-Gene Conditions
Single-gene NIPT screens for genetic conditions are related to changes in just one gene. Like standard NIPT, it relies on cell-free DNA to predict the risk that your baby will have a certain genetic condition. Single-gene NIPT looks for conditions that would not be screened for on a standard NIPT, but that can have significant impacts on a baby’s physical and mental development. For that reason, single-gene NIPT is often done in addition to a standard NIPT.
The benefit of having single-gene NIPT as well as standard NIPT is that most single-gene conditions are difficult to detect with first trimester ultrasound findings. Some single gene disorders can be difficult to diagnose even after the baby is born. Like standard NIPT, single-gene NIPT requires only a blood draw and can be done as early as nine weeks of pregnancy.
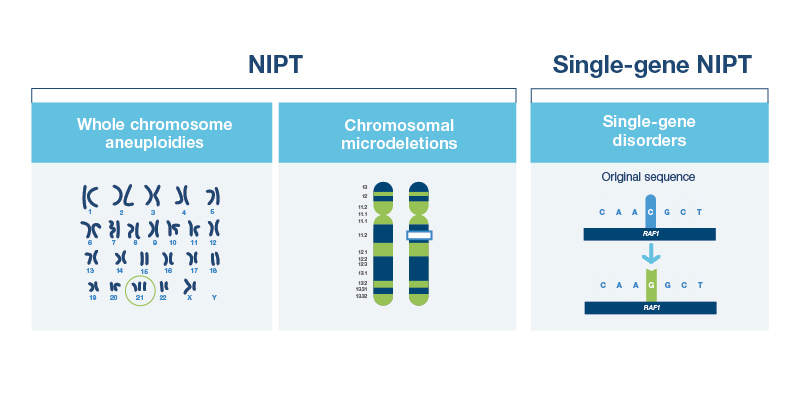
Some of the conditions that single-gene NIPT can screen for:
- CHARGE syndrome
- Apert syndrome
- Rett syndrome
- Noonan syndrome
- Osteogenesis imperfecta
Individually, these conditions are relatively rare, however the combined incidence of these conditions is greater than that of Down syndrome.18-19
Who Should Get Single-Gene NIPT?
Like all NIPT, single-gene NIPT is safe for all pregnant individuals and poses no risk to your baby. However, because each condition it screens for is relatively rare, many providers only offer single-gene NIPT if you request additional testing or have a known risk factor such as:
- A male partner over 40 years old
- Certain ultrasound findings including shortened long bones
- A family history of certain hereditary conditions19
If you think you are at high risk for having a baby with a single-gene condition, or want to have as much early information as possible, talk to your provider or genetic counselor about single-gene NIPT.
Vistara™ for Comprehensive Single-Gene NIPT
Vistara is the single-gene NIPT from Natera that screens for 25 known skeletal, cardiac, and neurological conditions across 30 genes. The conditions it screens for are associated with at least one of the following developmental challenges:
- Need for surgical/medical intervention
- Cognitive differences
- Impacted quality of life
In clinical validation studies, Vistara had over 99% sensitivity and over 99% specificity for the conditions screened, making it a highly accurate choice for single-gene NIPT.19
The Role of Genetic Counseling in Prenatal Genetic Screening
Before having any type of prenatal genetic screening, it’s always a good idea to discuss your questions and concerns with your provider. In some situations, your provider can refer you to a genetic counselor to help address your concerns.
A genetic counselor can be a valuable partner throughout your pregnancy journey by helping you make more informed decisions regarding genetic conditions. They can also refer you to appropriate services if needed.20 If your provider does not have a preferred genetic counselor or services are unavailable in your community, virtual genetic counseling services are available through many providers.
As part of Natera’s commitment to our patients, we provide complimentary information sessions with board-certified genetic counselors before and after testing. Our counselors are available to answer questions from 6 AM to 5 PM Pacific Time, Monday through Friday, excluding holidays. Please call 866-985-4336 and ask to speak with the on-call genetic counselor. You may also email gc@natera.com with questions.
Disclaimer
The tests described have been developed and their performance characteristics determined by the CLIA-certified laboratory performing the test. The tests have not been cleared or approved by the US Food and Drug Administration (FDA). Although FDA is exercising enforcement discretion of premarket review and other regulations for laboratory-developed tests in the US, certification of the laboratory is required under CLIA to ensure the quality and validity of the tests. CAP accredited, ISO 13485 certified, and CLIA certified. © 2022 Natera, Inc. All Rights Reserved.
References
- Zarocostas Z. Serious birth defects kill at least three million children a year. BMJ. 2006; 332(7536): 256. https://doi.org/10.1136/bmj.332.7536.256-b
- CDC. Facts about Down syndrome. Available from: https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html [Accessed January 20, 2022]
- Practice Bulletin No. 162: Prenatal diagnostic testing for genetic disorders. Obstet Gynecol. 2016 May;127(5):e108-e122. https://doi.org/10.1097/AOG.0000000000001405
- The American College of Obstetricians and Gynecologists. Amniocentesis frequently asked questions. Available from: https://www.acog.org/womens-health/faqs/amniocentesis [Accessed January 20, 2022]
- CDC. Genetic testing. Available from: https://www.cdc.gov/genomics/gtesting/genetic_testing.htm [Accessed January 20, 2022]
- Committee Opinion No. 691: Carrier screening for genetic conditions. Obstet Gynecol. 2017 Mar;129(3):e41-e55. https://doi.org/10.1097/AOG.0000000000001952
- Sinha R, Sarkar S, Khaitan T et al. Duchenne muscular dystrophy: Case report and review. J Family Med Prim Care. 2017 Jul-Sep; 6(3): 654-656. https://pubmed.ncbi.nlm.nih.gov/29417026/
- Broomfield J, Hill M, Guglieri M et al. Life expectancy in Duchenne muscular dystrophy. Neurology. 2021;97:e2304-2314. https://doi.org/10.1212/WNL.0000000000012910
- Bell CJ, Dinwiddie DL, Miller NA et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011;3(65):65ra4. https://doi.org/10.1126/scitranslmed.3001756
- Archibald AD, Smith MJ, Burgess T. et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genet Med. 2018;20, 513-523. https://doi.org/10.1038/gim.2017.134
- Westemeyer, M, Saucier J, Wallace J et al. Clinical experience with a carrier screening in a general population: support for a comprehensive pan-ethnic approach. Genet Med. 2020 Aug;22(8):1320-1328. https://doi.org/10.1038/s41436-020-0807-4
- Dhamankar R, DiNonno W, Martin KA et al. Fetal sex results of noninvasive prenatal testing and differences with ultrasonography. Obstet Gynecol. 2020 May; 135(5): 1198–1206. https://doi.org/10.1097/AOG.0000000000003791
- DiNonno W, Demko Z, Martin K, et al. Quality assurance of non-invasive prenatal screening (NIPS) for fetal aneuploidy using positive predictive values as outcome measures. J Clin Med. 2019;8(9):1311. https://doi.org/10.3390/jcm8091311
- Dar P, Jacobson B, MacPherson C. Cell-free DNA screening for trisomies 21, 18 and 13 in pregnancies at low and high risk for aneuploidy with genetic confirmation. Am J Obstet Gynecol. 2022. https://doi.org/10.1016/j.ajog.2022.01.019
- Dar P, Jacobson B, Clifton R et al. Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am J Obstet Gynecol. 2022. doi: https://doi.org/10.1016/j.ajog.2022.01.002
- McDonald-McGinn DM, Sullivan KE, Marino B et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1:15071. https://doi.org/10.1038/nrdp.2015.71
- Palmer LD, Butcher NJ, Boot E et al. Elucidating the diagnostic odyssey of 22q11.2 deletion syndrome. Am J Med Genet A. 2018 Apr;176(4):936-944. https://doi.org/10.1002/ajmg.a.38645.
- Snijders RJM, Sundberg K, Holzgreve W et al. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol. 1999;13:167-170. https://doi.org/10.1046/j.1469-0705.1999.13030167.x
- Zhang J, Li J, Saucier JB et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. Nat Med. 2019 Mar;25(3):439-447. https://doi.org/10.1038/s41591-018-0334-x
- CDC. Genetic counseling. Available from: https://www.cdc.gov/genomics/gtesting/genetic_counseling.htm [Accessed January 26, 2022]
