Patient Testimonial
With Signatera™, Jason has been able to manage his Stage IV CRC diagnosis, intervene early, fight smarter, and never stop planning for tomorrow. His team has had an edge in catching recurrence earlier—allowing for less invasive treatment plans and more peace of mind. Jason’s journey isn’t just about surviving—it’s about thriving. Watch his story.
Hear how Beth was able to get back to living after her stage III CRC treatment
How is the Signatera™ test performed?
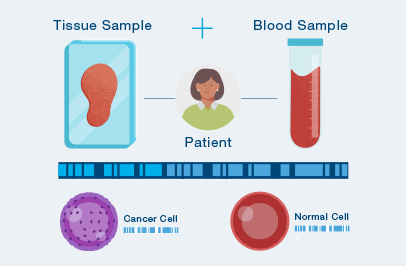
Personalized, tumor-informed assay
One-time, primary tissue sample and matched normal sample is required for whole exome or whole genome sequencing and personalized test design.

Ultrasensitive ctDNA detection
Signatera™ is designed to detect ctDNA of somatic and truncal variants to optimize sensitivity. Tumor-informed method enables filtering of CHIP mutations to decrease false positive rates.
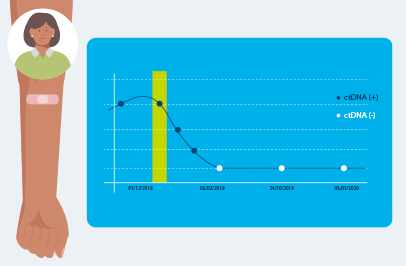
Optimized for longitudinal monitoring
Once the patient’s personalized test has been designed, only a blood sample is needed each subsequent time Signatera™ is ordered for the program or Surveillance program.
Peace of mind powered by Signatera™
Helping Patients Make Informed Decisions
For patients undergoing treatment for colon cancer, each scan can be a scary experience. Wondering if the treatments are working and the nagging fear of colorectal cancer recurrence can cause a great deal of anxiety and worry.
Signatera™ can help alleviate these concerns by making it much easier for doctors to assess response to treatment and watch for the earliest warning signs of recurrence. It is designed to detect molecular residual disease by looking for the distinct tumor DNA in each patient. This personalized, tumor-informed assay is optimized to provide accurate detection for each individual, making it a powerful source of information to help guide future care decisions.
Patient Resources
Questions regarding coverage?
Access Personalized Testing
Medicare Coverage
- Signatera™ is covered by Medicare for patients with stage II-IV and oligometastatic colorectal cancer.
- We welcome all insurance plans. Please refer to our list of in-network plans that we participate with, or call your insurance provider.
- We offer financial assistance programs for those that qualify.
More Information
- Find answers to your questions about eligibility, results, ordering, and more
- Don’t see your question? Contact us here.




