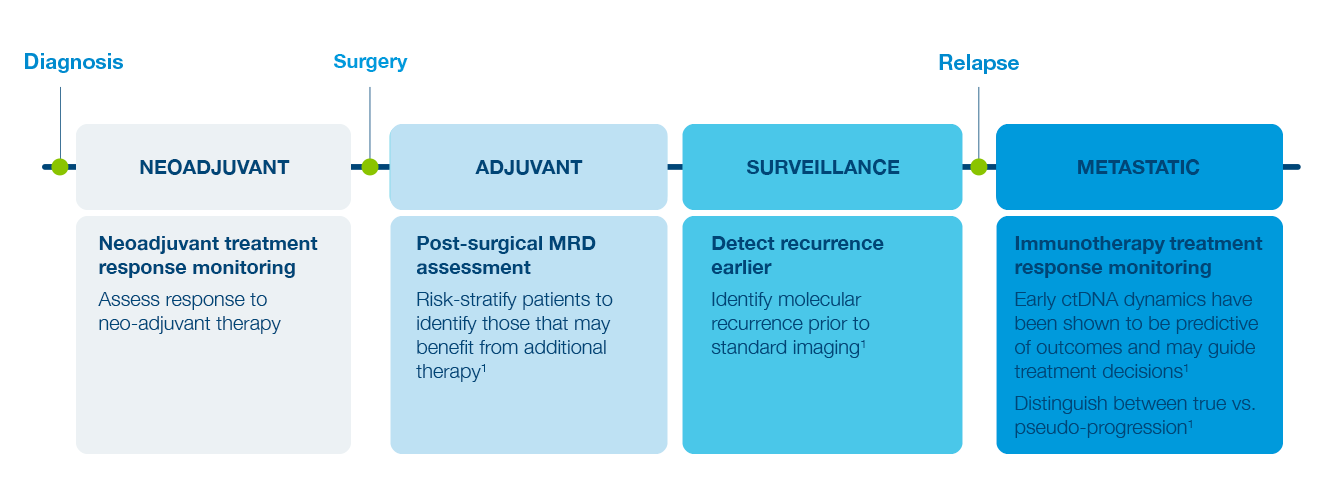
Inform Early Treatment Decisions
Identify High Risk Patients
Test for presence of MRD after surgery to risk-stratify patients1
>10x
Higher risk of recurrence for ctDNA positive patients1
Detect Recurrence Earlier
Detect recurrence prior to standard imaging1
3 months
lead time in detecting recurrence compared to PET/CT1
Predict Response to Treatment
Track changes in ctDNA levels to evaluate treatment response1
6 weeks
To identify patients with poor prognosis due to increasing ctDNA during 1L IO treatment1

Signatera™ treatment response monitoring is covered by Medicare for any patient being treated with immunotherapy

ctDNA is now recommended by the National Comprehensive Cancer Network (NCCN®) for assessment of disease burden in both virus-positive and virus-negative Merkel Cell Carcinoma2
Inform Your Skin Cancer Treatment Approach With Signatera™
Explore Signatera™ data in Skin Cancer
Adjuvant Melanoma and Surveillance
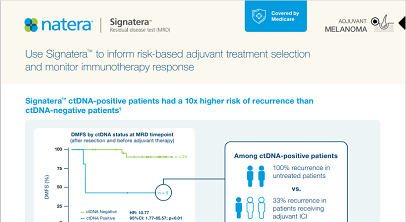
Use Signatera™ to inform risk-based adjuvant treatment selection and monitor immunotherapy response
Signatera™ ctDNA-positive patients had a 10x higher risk of recurrence than ctDNA-negative patients1
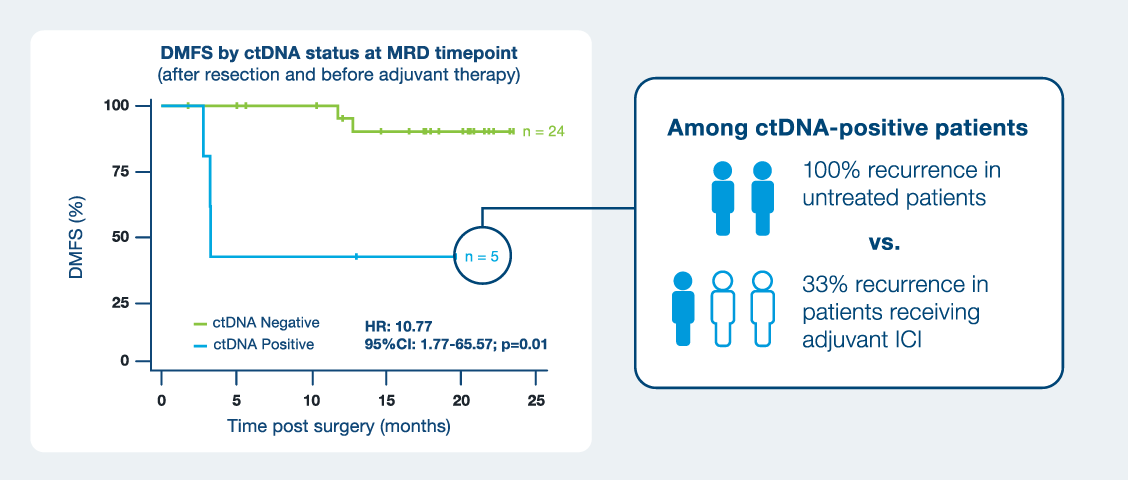
- ctDNA-positivity was associated with significantly shorter DMFS (HR=10.77; p=0.01)
- Outcomes were improved in ctDNA-positive patients who were treated with adjuvant ICI.
- ctDNA-positivity at week 6 of adjuvant ICI therapy was significantly associated with an inferior DMFS (HR:34.54,P<0.0001)
On-treatment ctDNA dynamics were significantly associated with DMFS1
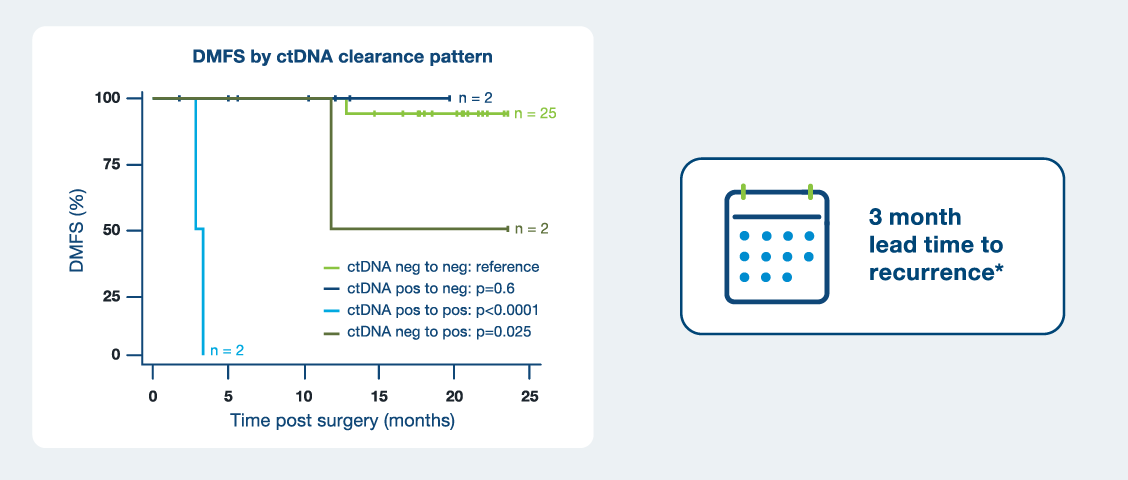
- Patients who became or remained ctDNA negative experienced superior outcomes than patients who converted to or were persistently ctDNA positive
- Signatera™ provided a lead time to recurrence of 3 months vs. standard imaging
* Average time to recurrence by PET/CT: ~9 months vs. average time to recurrence by ctDNA: ~6 months
DMFS = Distant metastasis-free survival; ICI = immune checkpoint inhibitor; MRD =
Molecular residual disease; ctDNA = Circulating tumor DNA
Metastatic Melanoma
Use Signatera™ ctDNA dynamics to inform earlier treatment decisions in metastatic melanoma patients
Early on-treatment ctDNA dynamics were predictive of PFS in metastatic melanoma patients receiving 1st line ICI treatment1
At week 6, Signatera™ identified that patients with increasing ctDNA had a 18x higher risk of progression than ctDNA-negative patients
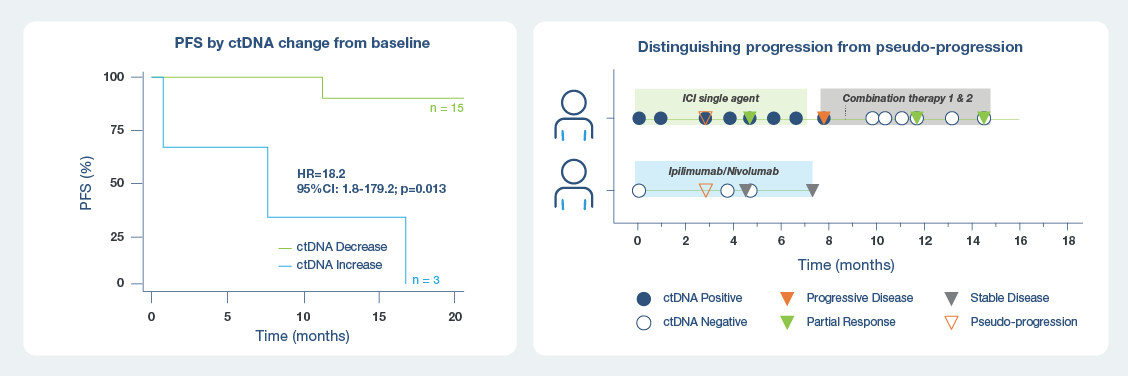
- Patients with any increase in ctDNA levels from baseline by week 6 of 1st Line ICI treatment (monotherapy and combination ICIs) had a significantly shorter PFS (HR: 18; p=0.013).
- Signatera™ was able to help distinguish between true vs pseudo-progression
Merkel Cell Carcinoma
Use Signatera™ to inform risk-based surveillance strategy
Circulating tumor DNA (ctDNA) is now recommended by the National Comprehensive Cancer Network (NCCN®) for assessment of disease burden in both virus-positive and virus-negative Merkel Cell Carcinoma (MCC)2
- Merkel Cell Carcinoma represents the first instance of an NCCN recommendation for ctDNA MRD testing in a solid tumor cancer, a significant step forward in improving patient management2
- ctDNA was recently added as an NCCN Category 2A recommendation to the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for both virus-positive and virus-negative patients across all stages2
- Testing is recommended as part of an additional workup during the initial diagnosis and as part of routine follow-up surveillance2
- The NCCN Guidelines® indicated that testing is “often obtained every 3 months.”2

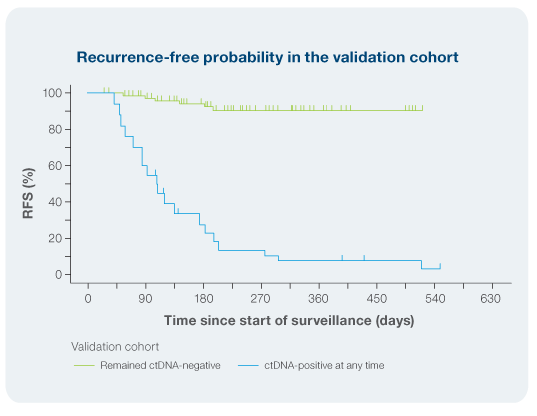
Akaike T. et al: Patients who were Signatera-positive had up to a 20x higher risk of recurrence (HR:20)3
- Signatera™ demonstrated 95% sensitivity for detecting disease at enrollment.3
- ctDNA-positive patients had >70% likelihood of recurrence within 6 months of a positive test, while ctDNA-negative patients had a <5% likelihood of recurrence within 3 months of a negative test3
- Signatera™ demonstrated a 94% PPV and 93% NPV for recurrence respectively3
Provide Confidence for Patients With Skin Cancer
After Jeffrey started dual immunotherapy for his metastatic melanoma, Signatera™ showed a dramatic drop in his ctDNA levels and helped provide assurance that his treatment was working.
Watch Jeffrey’s story to learn how his oncology team used Signatera™ to help guide his care and restore his confidence in his future.
MRD Testing for Melanoma: The Next Step in Recurrence Prevention
Watch this webinar from the Melanoma Research Foundation to learn more about how MRD is being used to help manage melanoma.
*Clicking this button will open it on YouTube.com
Learn More About Signatera in Skin Cancers
Is Signatera™ right for your patients with skin cancer?
References
1Eroglu Z, Krinshpun S, Kalashnikova E, et al. Circulating tumor DNA based molecular residual disease detection for treatment monitoring in advanced melanoma patients. Cancer (2023). https://doi.org/10.1002/cncr.34716
2Referenced with permission from the NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) for Merkel Cell Carcinoma V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed April 1st, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
3Akaike T, Thakuria M, Silk AW, et al. J Clin Oncol. 2024