New Medicare Coverage: Signatera in NSCLC
Learn MoreNew Medicare Coverage: Signatera in NSCLC
Learn MoreDetect and Monitor ctDNA in NSCLC to Inform Treatment Strategy
Identify
High Risk Patients
Test for presence of MRD after definitive therapy to assess prognosis1-2
27x
Higher risk of recurrence
for ctDNA positive patients1
Detect
Recurrence Earlier
Detect recurrence before
radiographic imaging1-2
5-7 months
Median lead time over
radiographic recurrence1-2,8
Monitor Immunotherapy
Treatment Response
Track early changes in ctDNA levels to predict outcomes3
96% lower
Risk of death for patients who cleared their ctDNA by week 9 compared to patients with increasing ctDNA3
Better Biomarkers are Needed to Optimize NSCLC Patient Care
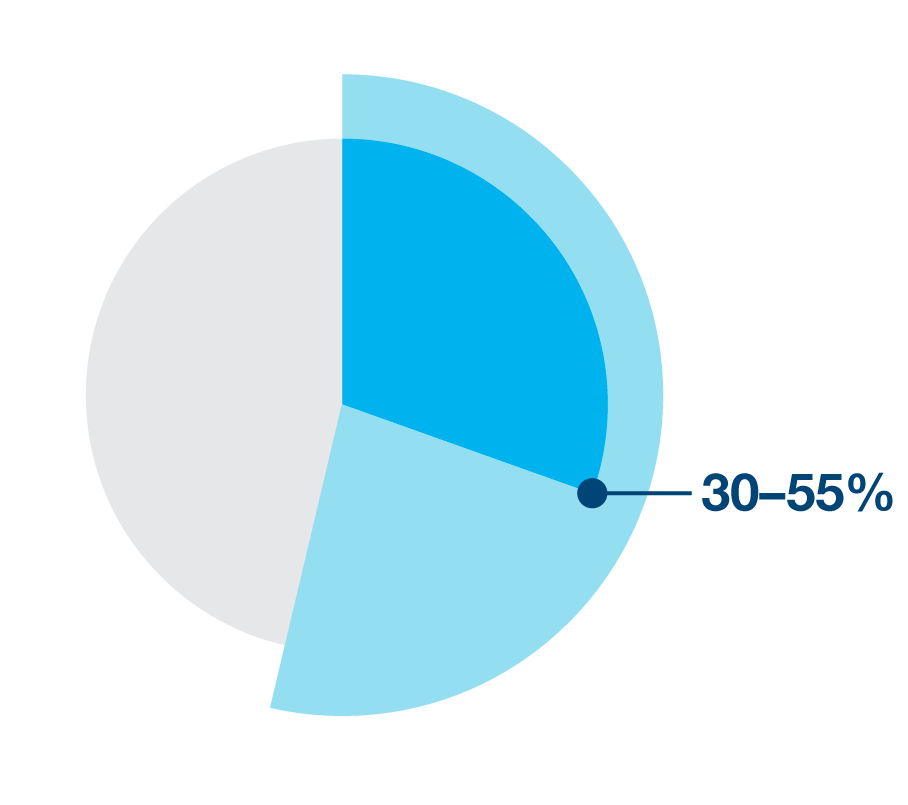
30-55% of NSCLC patients will develop disease recurrence post-definitive treatment (surgery; chemo-radiation)4
Guidelines for stage IB-IIA patients provide the option of either adjuvant systemic therapy or observation alone5
Early detection of recurrence with less tumor burden may enable local treatment with curative-intent, potentially improving outcomes6
Inform Your Lung Cancer Treatment Approach With Signatera™
Monitoring circulating tumor DNA (ctDNA) with Signatera™ across the treatment journey can answer important clinical questions and inform treatment decisions.
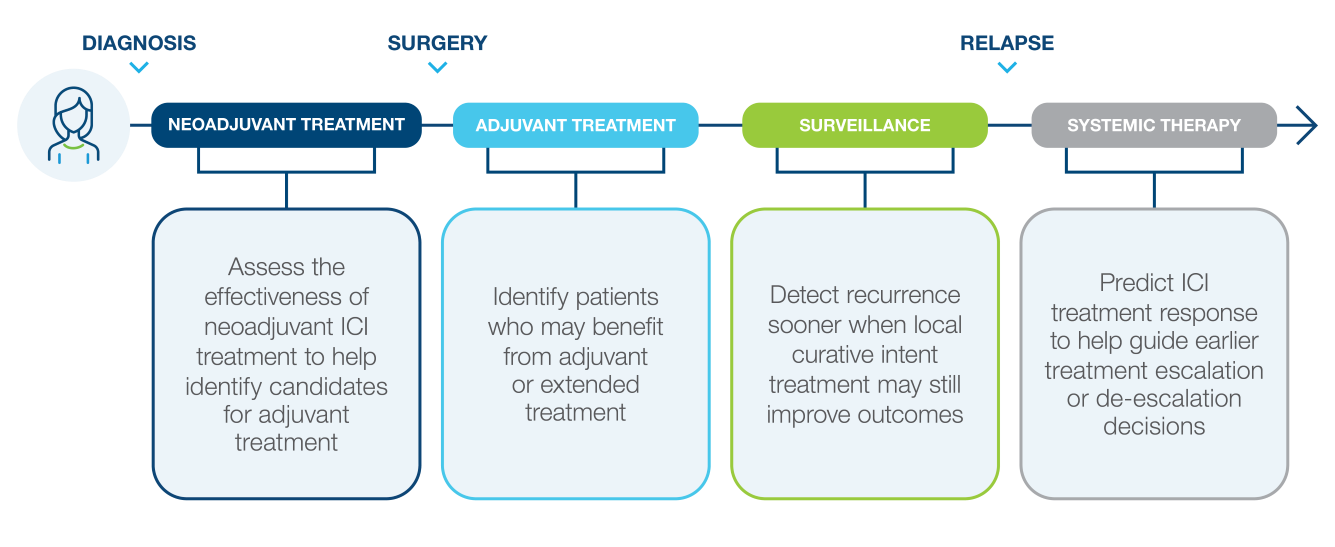
Identify High Risk Patients and Detect Recurrence Sooner
Signatera™ was evaluated in Martin et al 2024, a study in patients with stage I-II resectable NSCLC after surgical resection and during surveillance1
- Post-operative surveillance strategies were altered in 100% of ctDNA-positive patients (earlier radiographic imaging), and patients with PET scans positive for malignant features received early referrals for treatment
- 98% NPV (negative predictive value)
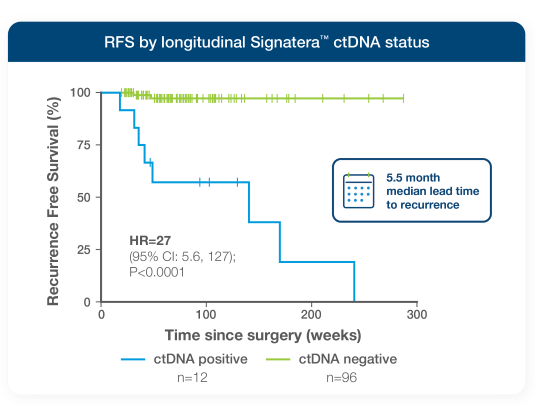
- Signatera™ detected relapse with a 5.5-month median lead time to imaging-confirmed relapse
- 27x higher risk of recurrence for ctDNA-positive patients
Signatera™ Genome data was presented at ASCO 2025 with a subcohort of 76 stage I-III resectable NSCLC patients7
- Signatera™ Genome detected distant, extracranial recurrence with 91% longitudinal sensitivity and 100% specificity
- Single time point sensitivity and NPV for distant, extracranial recurrence were 75% and 96%, respectively, the highest MRD performance in lung cancer to date
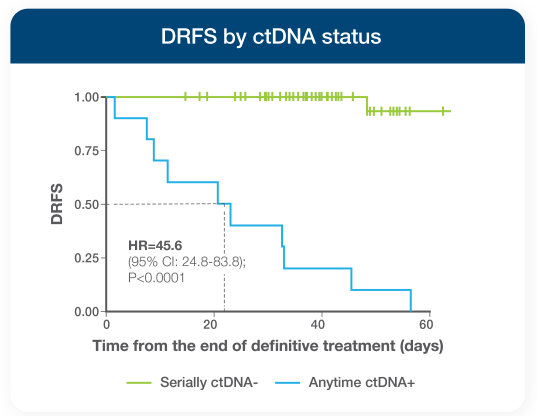
- Patients who were serially ctDNA-negative experienced 100% 24-month DRFS vs just 40% for anytime ctDNA-positive patients
- Signatera™ Genome detected recurrence a median of >7 months before standard surveillance
- Every patient who tested ctDNA-positive with Sigantera™ Genome went on to recur during follow-up
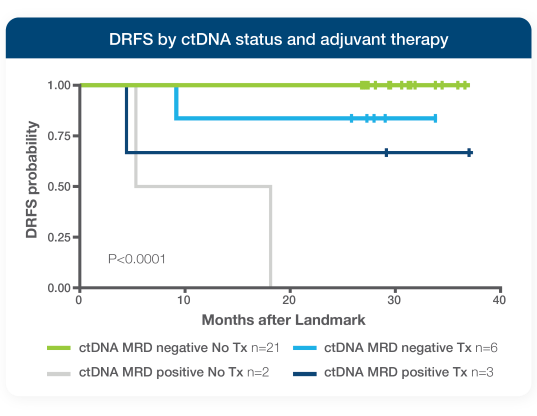
Predict benefit from adjuvant treatment7,8
- Patients who were ctDNA-positive after surgery received significant benefit from adjuvant therapy8
- Patients who were ctDNA-negative after surgery received no benefit from adjuvant therapy, achieving 100% 12-month DRFS despite receiving no additional treatment8
Evaluate Response to ICI Treatment in NSCLC
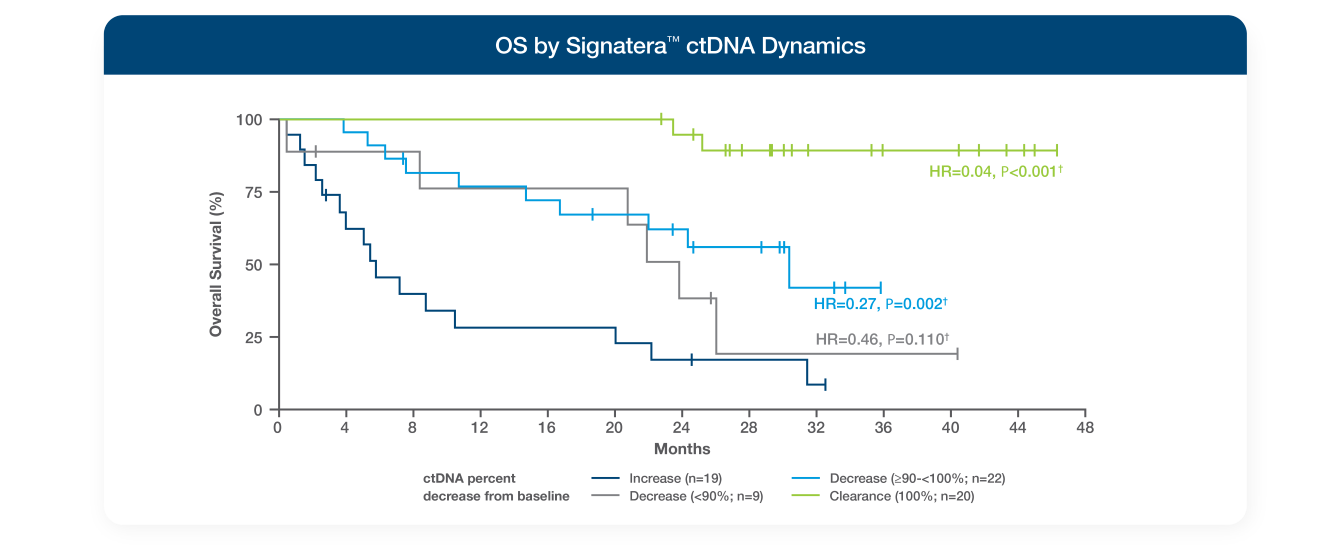
Signatera™ was evaluated in the EMPower Lung-1 trial, a prospective study in patients with advanced or metastatic NSCLC randomized to immune checkpoint inhibitor (ICI) vs chemotherapy for 1L treatment3
- Rising ctDNA by week 3 or lack of ctDNA clearance by week 9 identified patients experiencing inferior clinical outcomes
- ctDNA deep decrease (>90%) and clearance were associated with significantly improved OS
- Composite ctDNA & RECIST assessment may improve prediction of OS benefit from ICI
Watch to Learn More About Signatera™
See the Latest Data and How Signatera is Used to Personalize NSCLC Patient Care with Oleg Gligich, MD and Michael Krainock, MD, PhD
Review the EMPOWER-Lung 1 trial with Natalie Vokes, MD
Reassure Your Patients Living With Lung Cancer
After JoAnn was diagnosed with lung cancer, Signatera™ detected a rise in her ctDNA levels, alerting her oncologist to a progression that was not scannable.
Watch JoAnn’s story to learn how she and her oncologist added Signatera™ testing to their toolbelt to help closely monitor her cancer.
“I want to know. The more you know, the better off you are.” – JoAnn, living with lung cancer

Do More With Less Tissue
One tumor sample – two tests
Lung Cancer Resources

The benefits of using ctDNA over traditional scans to assess responses to ICI
Dr. Natalie Vokes shares thoughts on how ctDNA can be used in addition to scans in NSCLC

Signatera™ in Lung Cancer
Learn about Signatera™ for risk-stratification, surveillance, and immunotherapy response monitoring

Patient Case Study
Read how Signatera™ detected a rise in ctDNA levels during immunotherapy, informing the decision to pivot to a combination therapy approach.
Ready to try Signatera™ for your lung cancer patients?
*OS = Overall Survival
References
1Martin TK, Dinerman A, et al. Early Real-World Experience Monitoring Circulating Tumor DNA in Resected Early-Stage Non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg. 2024. doi: 10.1016/j.jtcvs.2024.01.017.
2Lebow E, et al. ctDNA-based detection of molecular residual disease in stage I-III non-small cell lung cancer patients treated with definitive radiotherapy. Front. Oncol. 2023,13:1253629.
3Vokes N, Gandara D, et al. Circulating Tumor DNA (ctDNA) Dynamics and Survival Outcomes in Patients with Advanced NSCLC and High (greater than 50%) PD-L1 Expression, Randomized to Cemiplimab vs Chemotherapy. Presented at ASCO Annual Meeting, Chicago, IL, June 2023.
4Uramoto H, Tanaka F. Translational Lung Cancer Research. 2014;3:242–249
5NCCN Clinical practice guidelines in Oncology for NSCLC. V1 2024
6Gomez DR, et al. J Clin Oncol 2019;37:1558-1565.
7George M, et al. Poster presented at the 2025 ASCO Annual Meeting, Chicago, IL, June 2025.
8Natera Data on File.