Inform Clinical Challenges in Colorectal Cancer
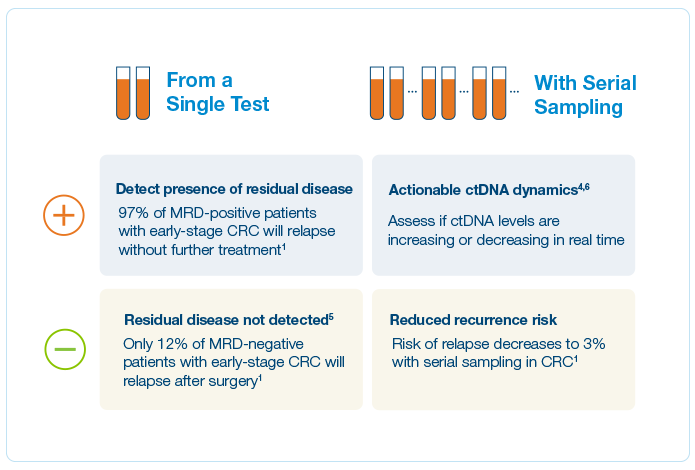
Identify High Risk Patients
80%
Of CRC patients are cured by surgery alone — knowing which patients would benefit from ACT is often unclear1
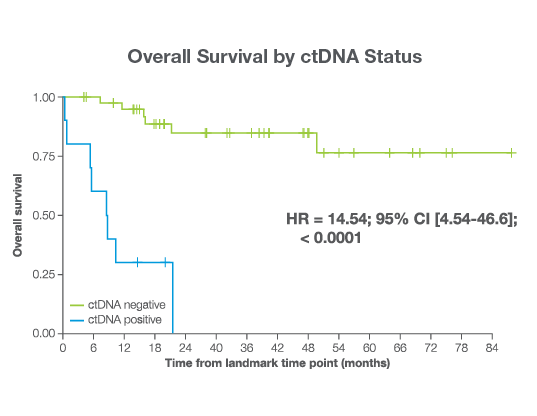
Predict Overall Survival
36-month OS
96%
in ctDNA negative patients vs. 71.8% in ctDNA positive patients2
ctDNA clearance and survival
100%
MRD-positive patients who achieved sustained clearance had 100% OS at 24 months2
Get Started
Powering Personalized Decisions in Early and Late Stage CRC
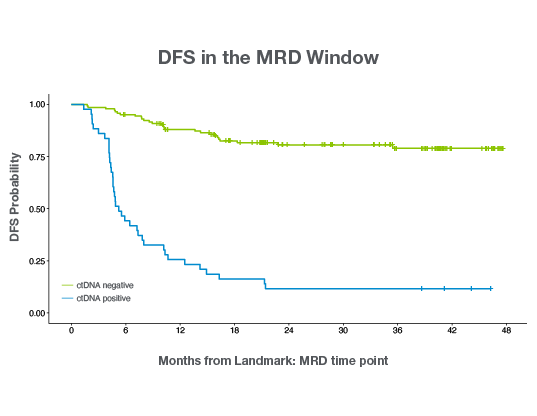
Signatera™ status post-operatively is highly prognostic of disease recurrence
The latest GALAXY findings from the CIRCULATE-Japan trial analyzed >2,240 patients with stage II– IV colorectal cancer including 36M-DFS and 24M- OS data, demonstrating the importance of post-surgical ctDNA analysis and its association with long term survival:2,3
- Signatera™ MRD status predicted overall survival: Patients who tested Signatera™-positive after surgery had significantly worse overall survival (OS) compared to those who were Signatera™-negative.
- Signatera™ MRD status identified adjuvant chemotherapy benefit: High-risk stage II and stage III–IV patients who were Signatera™-positive post-surgery and received ACT showed improved OS.
- Signatera™ positivity was shown to be the strongest predictor of recurrence: Post-operative Signatera™ positivity was the most significant prognostic factor associated with decreased disease-free survival (DFS).
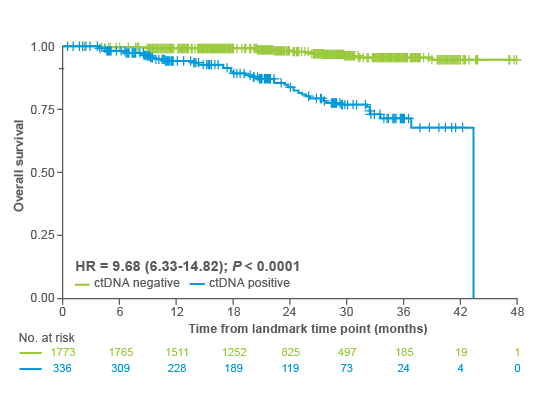
Predict which CRC patients may benefit from treatment escalation
The CALGB readout from ASCO GI analyzes >1,000 patients with stage III colorectal cancer randomized to receive FOLFOX (+/-) celecoxib:
- Signatera™-positive patients treated with both chemotherapy and celecoxib showed a 40% improvement in overall survival compared to chemotherapy alone5.
- No benefit from celecoxib was observed in Signatera™-negative patients.
- Patients who were Signatera™-positive after surgery were 7x more likely to recur and >6x more likely to have decreased OS than patients who were Signatera™-negative.
Natera Oncology can help support this therapy recommendation:
- Altera™ tumor genomic profiling for patient selection + Signatera™ MRD testing
- Altera™ detects PI3K pathway mutations (PIK3CA, PTEN, PIK3R1), which guide NSAID therapy decisions
With a single sample, you can get molecular residual disease (MRD) and tumor genomic profiling from the same tumor sample, enabling you to select the right patients for additional therapy and track their MRD status over time.
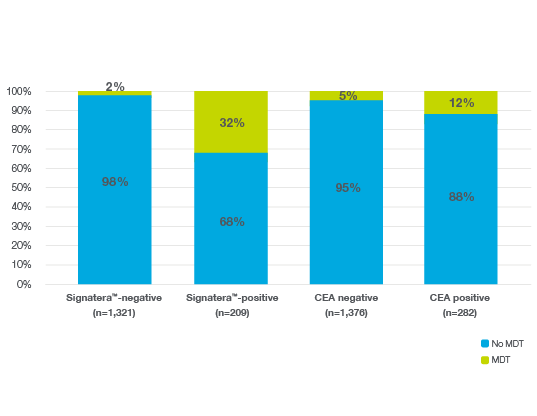
Identify which patients will benefit from increased surveillance
- Signatera™ identified more patients eligible for curative-intent MDT during surveillance by detecting recurrence at the molecular level, when patients are still candidates for local therapy6
- Signatera™-positive patients had a 35x higher risk of recurrence: Signatera™ detected relapse 6 months before recurrence and showed a 78% lower recurrence risk with ACT in high-risk stage II/III CRC.6
- Signatera™-positive patients – up to 20x more likely to receive MDT compared to Signatera™-negative patients, outperforming CEA6
Discover the Colorectal Cancer Data
- Signatera™ MRD testing in colorectal cancer is validated across treatment settings.
-
Neoadjuvant Response Monitoring
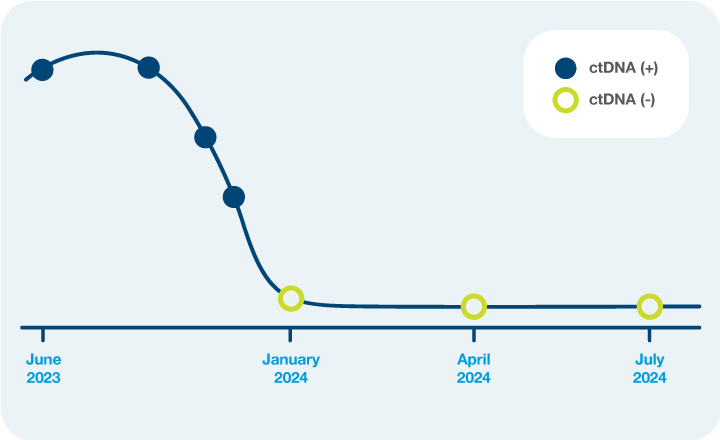
- Monitor neoadjuvant response with serial Signatera™ testing.
- Identify low risk patients who are ctDNA-negative to potentially support a nonsurgical “watch and wait” approach.
-
Post-Surgical MRD Assessment
-
Recurrence Monitoring
-
ctDNA clearance
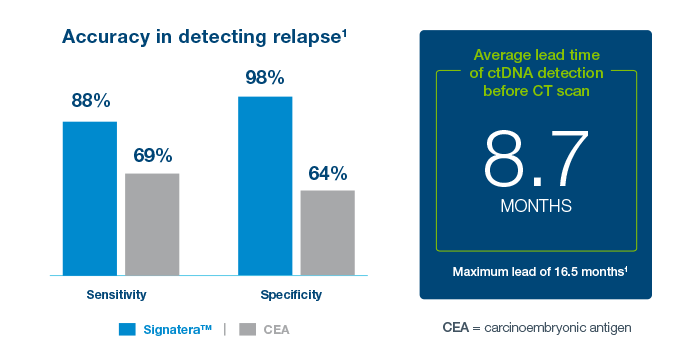
Clinical Application: What’s the value of serial testing in the surveillance setting?
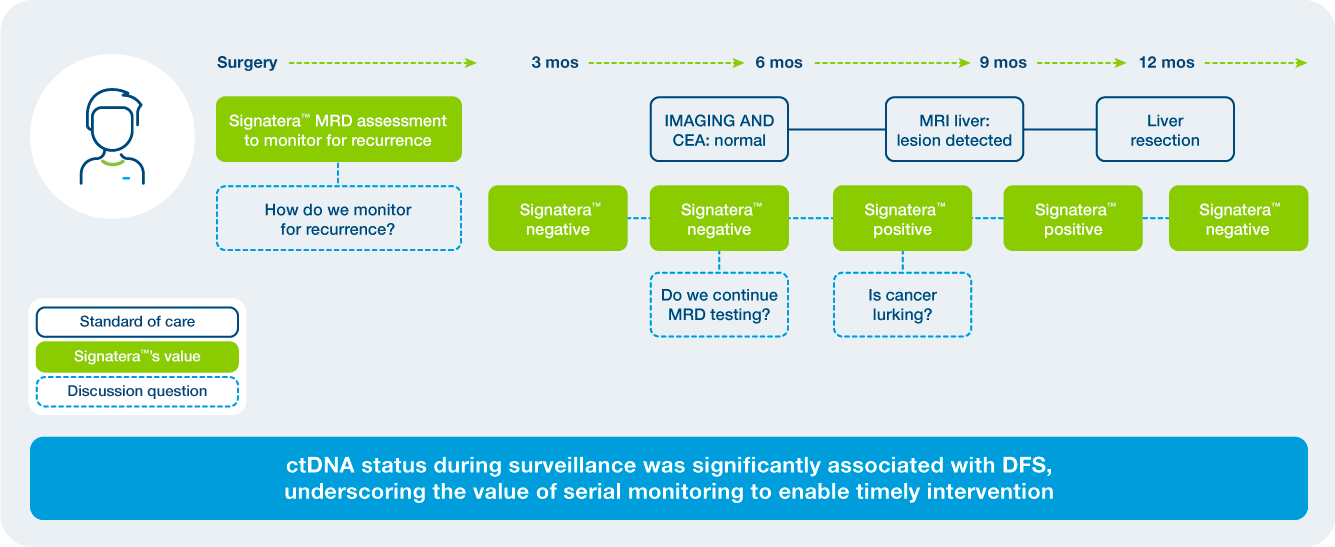
Enabling time to process and make informed plans
“Signatera fired the warning shot that something was not right. Together with my HCP we had the opportunity to take the time to consider the options. She put the choice on me on what to do with a positive test result and it enabled me to choose to take a more aggressive approach.”
Keith, CRC patient
Expanding MRD testing to more patients with colorectal cancer
Latitude™ is a tissue-free, blood-based, residual disease test (MRD) that delivers fast and reliable results without the need for tumor tissue. Latitude™ is built using a targeted panel, composed of differentially methylated regions specifically for colorectal cancer (CRC). Latitude™ offers strong performance, with high sensitivity and specificity for detecting residual disease in CRC patients.7
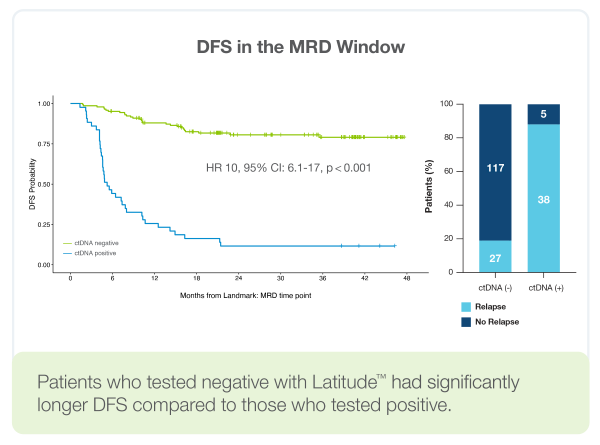
- Patients who tested negative with Latitude™ had significantly longer DFS compared to those who tested positive. HR ratio of 10, p<0.001
- 58% MRD time point sensitivity
- 81% longitudinal sensitivity (84% colon only)
- 97% Sample Level Specificity
- 4.6 months lead time before radiographic relapse
A oncology portfolio that supports actionable molecular insights
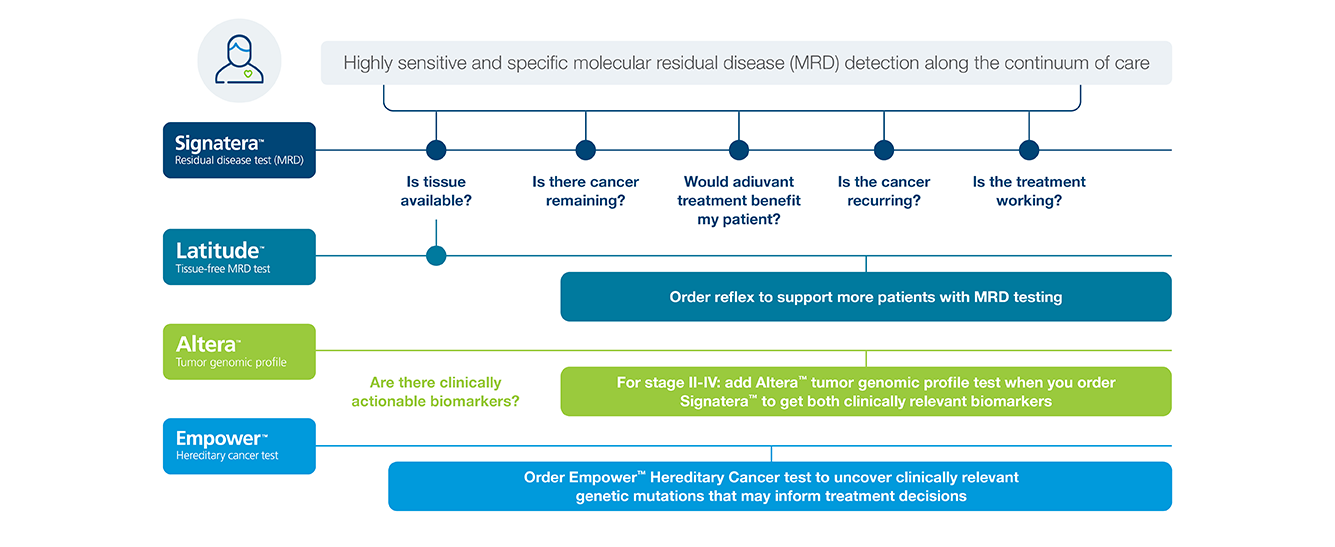
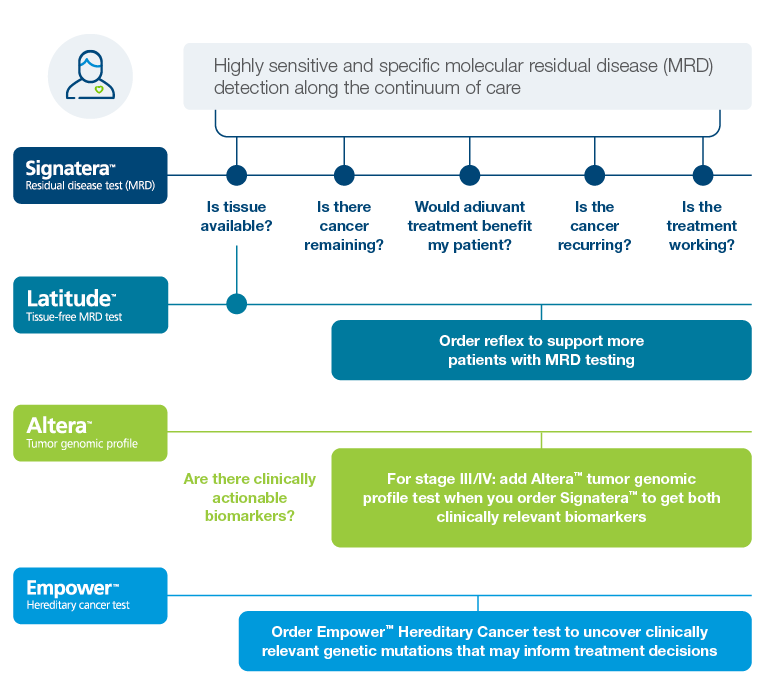
Gastroesophageal
Identify gastroesophageal cancer patients at high risk of recurrence using Signatera™
The PLAGAST study analyzes 62 patients with locally advanced, resectable gastroesophageal cancer to evaluate Signatera™ ctDNA dynamics from neoadjuvant therapy through the MRD window (4–6 weeks after surgery):
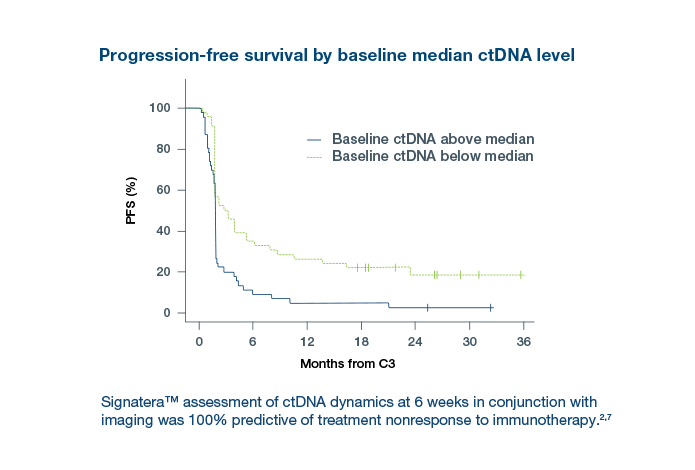
- Signatera™ status predicts poor outcomes across treatment time points: Signatera™ positivity during neoadjuvant therapy (NAT), post-NAT, and post-surgery was consistently associated with inferior recurrence-free survival (RFS) and overall survival (OS).8
- Longitudinal Signatera™ dynamics strongly stratify patient outcomes: Patients with early ctDNA clearance during NAT had the best survival.8
- Signatera™ status outperforms traditional clinicopathological factors: In multivariate analysis, Signatera™ status was the most significant independent risk factor for both RFS and OS, more predictive than tumor stage, nodal
status, or TRG score.
Pancreatic Cancer
Identify pancreatic cancer patients at high risk of recurrence using Signatera™
This real world study analyzes 298 patients with pancreatic ductal adenocarcinoma to evaluate the prognostic value of Signatera™ across the perioperative and surveillance settings:
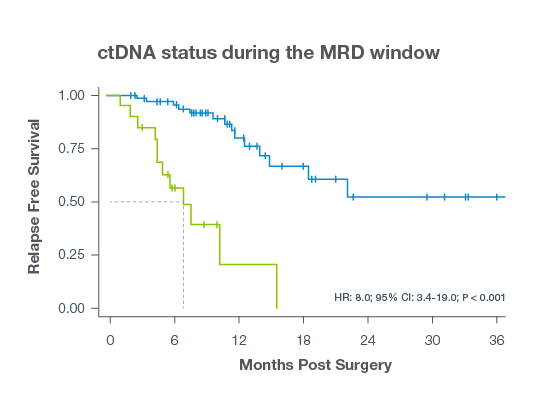
- Signatera™-positivity after surgery predicts early recurrence In the MRD window (2–12 weeks post-surgery), Signatera™-positive patients had a median DFS of 6.4 months versus 33.3 months for Signatera™-negative patients.9
- In surveillance Signatera™ positivity is the strongest predictor of recurrence Signatera™ positivity during surveillance was associated with significantly shorter DFS, with a median of 11.4 months.9
- Signatera™ detection at any time postoperatively was found to be the most significant prognostic factor for recurrence (HR = 24.28, P < 0.001)9
Hepatocellular Carcinoma
Catch recurrence sooner while intervention is still possible
The real world study analyzes 125 HCC patients across four clinical scenarios to assess the utility of Signatera™ after liver transplantation, surgery, or during systemic therapy:
- Signatera™ detected recurrence earlier than imaging or AFP In post-surgical patients, Signatera™ detected relapse 7.9 months earlier than clinical recurrence, outperforming AFP (median lead time 2.2 months).10
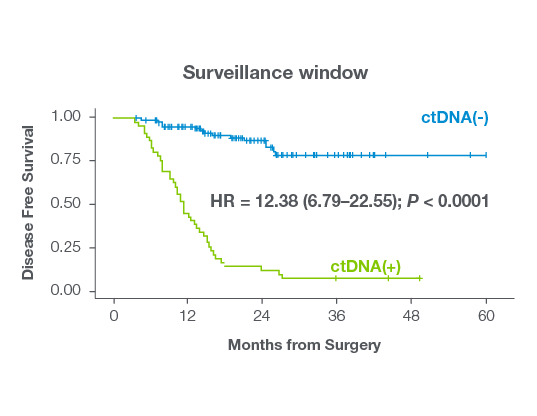
- Signatera™ demonstrated strong prognostic utility All patients with Signatera™ positivity after resection relapsed (MRD: HR 7.2, P < 0.0001, Surveillance: HR = 18.0, P < 0.0001).10
- Post-transplant Signatera™ negativity strongly predicts durable remission All liver transplant patients who were Signatera™- negative in the MRD window remained recurrence free, with only one late relapse yielding a 98% negative predictive value.
Biliary Cancers
Identify biliary cancer patients at high risk of recurrence using Signatera™
The real world study analyzes 167 patients with resected stage I–III biliary tract cancer to evaluate Signatera™ ctDNA as a prognostic biomarker during the post-surgical MRD and surveillance windows:
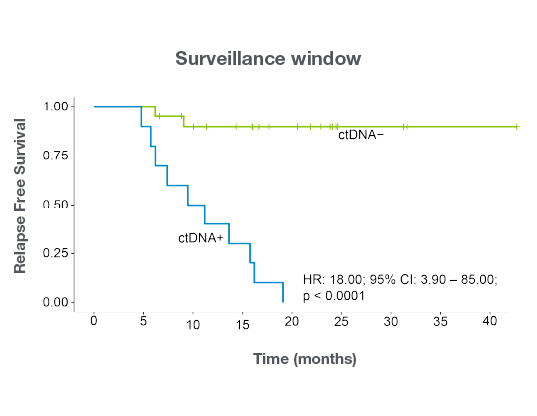
- Post-surgical ctDNA positivity predicts recurrence Signatera™-positivity in the MRD window was associated with worse relapse-free survival and overall survival.11
- Surveillance ctDNA status outperforms traditional biomarkers Signatera™-positivity during surveillance was the strongest predictor of relapse-free survival, while CA19-9 and CEA showed no prognostic value.11
- Integrating Signatera™ into post-surgical care to help inform adjuvant treatment decisions and personalize surveillance in BTC.11
Anal Cancer
Identify biliary cancer patients at high risk of recurrence after chemoradiation using Signatera™
In a prospective real-world study of anal cancer, Signatera™ ctDNA reliably predicted treatment response, relapse risk, and survival.
- Early Signatera™ clearance is linked to significantly improved PFS, OS, and locoregional control, establishing ctDNA as a prognostic biomarker.12
- Post-treatment Signatera™ positivity signals high risk of failure, with molecular recurrence guiding reflex clinical evaluation for early recurrence detection.12
- Signatera™ surveillance is highly predictive: 0% recurrence with sustained clearance vs. 100% treatment failure when ctDNA is detectable.12
Covered by Medicare for multiple solid tumor indications
Learn More About Signatera™ in Gastrointestinal Cancers
Ready to try Signatera™ for your colorectal cancer patients?
References
1Reinert T, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncology. 2019;5(8):1124–113. DOI: 10.1001/jamaoncol.2019.0528.
2Kotani D, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nature Medicine. 2023;29(1).
3Nakamura Y, Watanabe J, Akazawa N, et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nature Medicine. 2024.
4Bratman SV, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1(9):873–881. DOI: 10.1038/s43018-020-0096-5.
5Nowak JA, et al. Prognostic and predictive role of ctDNA in stage III colon cancer treated with celecoxib: Findings from CALGB/SWOG 80702. Presented at ASCO GI, 2025.
6Dasari A, et al. Clinical utility of including circulating tumor DNA monitoring in standard of care colorectal cancer surveillance. Presented at ESMO Gastrointestinal Cancer Annual Meeting, 2025.
7Nakamura Y, et al. Clinical validation of a tissue-free colorectal cancer test for the detection of molecular residual disease by circulating tumor DNA. Poster presented at ESMO GI, Barcelona, Spain. July 2025. Poster 93P.
8Zaanan A, Didelot A, Broudin C, et al. Longitudinal ctDNA analysis during treatment of resectable gastric and GEJ cancer: the PLAGAST study. Nature Communications. 2025;16:6815.
9Botta M, et al. Tumor-informed ctDNA predicts recurrence and survival in resected pancreatic cancer. The Oncologist. 2024. DOI: 10.1093/oncolo/oyae155.
10Abdelrahim M, et al. The feasibility of personalized and tumor-informed ctDNA assay for early recurrence detection in patients with hepatocellular carcinoma. JCO Precision Oncology, 2025.
11Abdelrahim M, et al. Real-world ctDNA analysis in resected Stage I–III biliary tract cancer. Poster #420. Presented at ASCO, 2025.
12Bercz JP, et al. Circulating tumor DNA as an early response indicator in anal squamous cell carcinoma treated with chemoradiation. Presented at American Society of Clinical Oncology, Chicago, May 30–June 3, 2025.