Signatera™ for Bladder Cancer
Signatera™ monitors ctDNA for bladder cancer and can help:
-
risk-stratify patients with MIBC1,2
-
predict disease recurrence early, before traditional imaging methods2
-
predict and monitor treatment response to inform clinical decisions3
Predict Adjuvant Treatment Benefit
ctDNA-positive patients treated with atezolizumab had a
42%
increase in disease-free survival1
Detect Recurrence Earlier
Signatera predicted recurrence up to
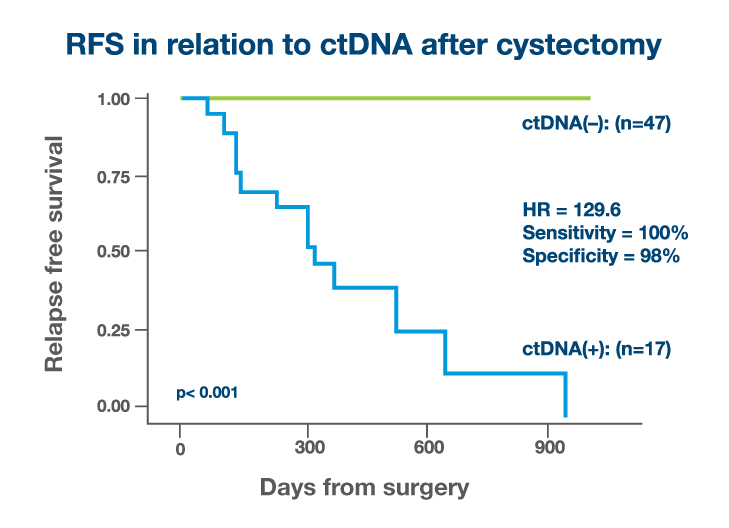
245 days
before clinical recurrence (median=96 days)2
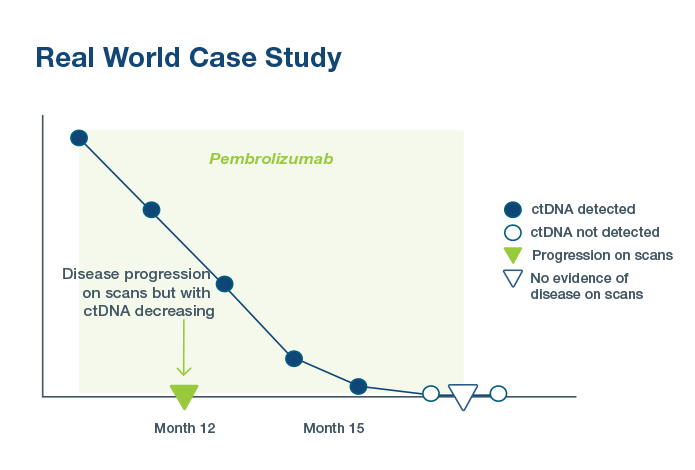
Predict Treatment Response
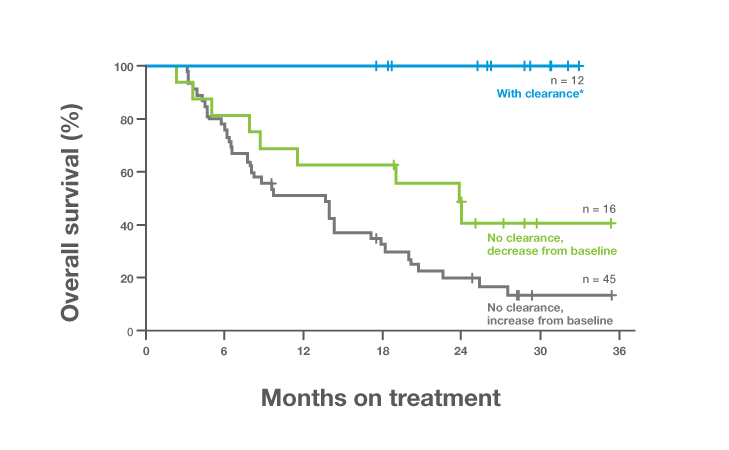
98% of metastatic patients with an increase in ctDNA
After 2 Cycles
did not derive an object response to immunotherapy treatment in a pan-tumor trial3
Get Started
Identify MIBC patients who may benefit from adjuvant therapy after cystectomy1,4
Signatera™ ctDNA positivity after cystectomy may predict adjuvant immunotherapy treatment benefit.1,4
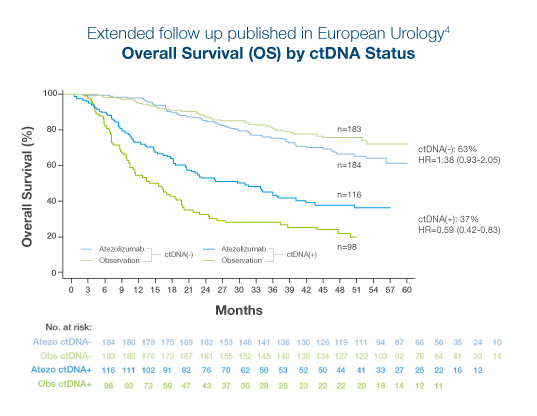
Extended follow-up from the Phase III, randomized IMvigor010 trial of atezolizumab vs observation in high risk adjuvant MIBC:4
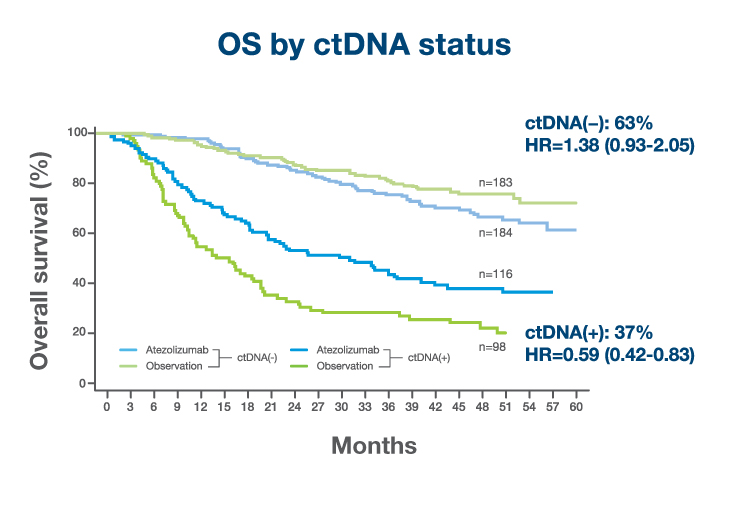
- >110% survival benefit observed in ctDNA-positive patients treated with atezolizumab (OS, HR 0.59).4
- No treatment benefit was observed in ctDNA-negative patients treated with atezolizumab (OS, HR 1.38)4
- 37% of patients were ctDNA-positive at C1D1 and ctDNA positivity predicted benefit from immunotherapy at 46.8-month median follow-up (OS, HR=0.59)4
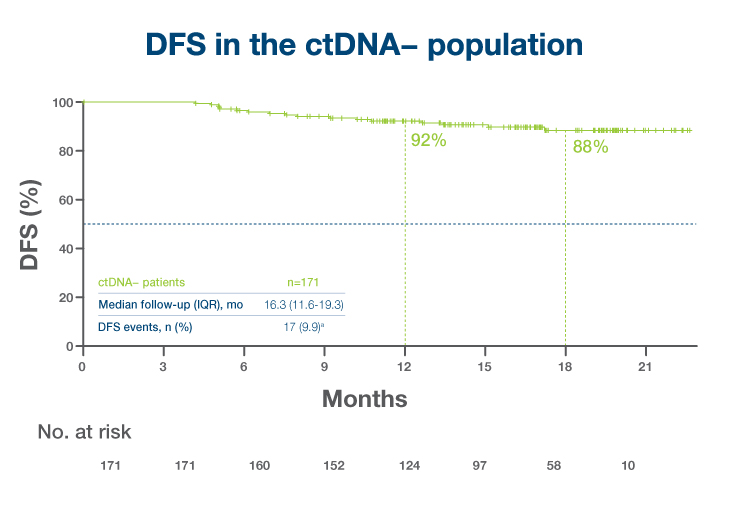
- >75% of patients with detectable ctDNA post-surgery in the observation arm recurred by 20 month follow up1
Get Actionable Insights Across the Bladder Cancer Care Journey
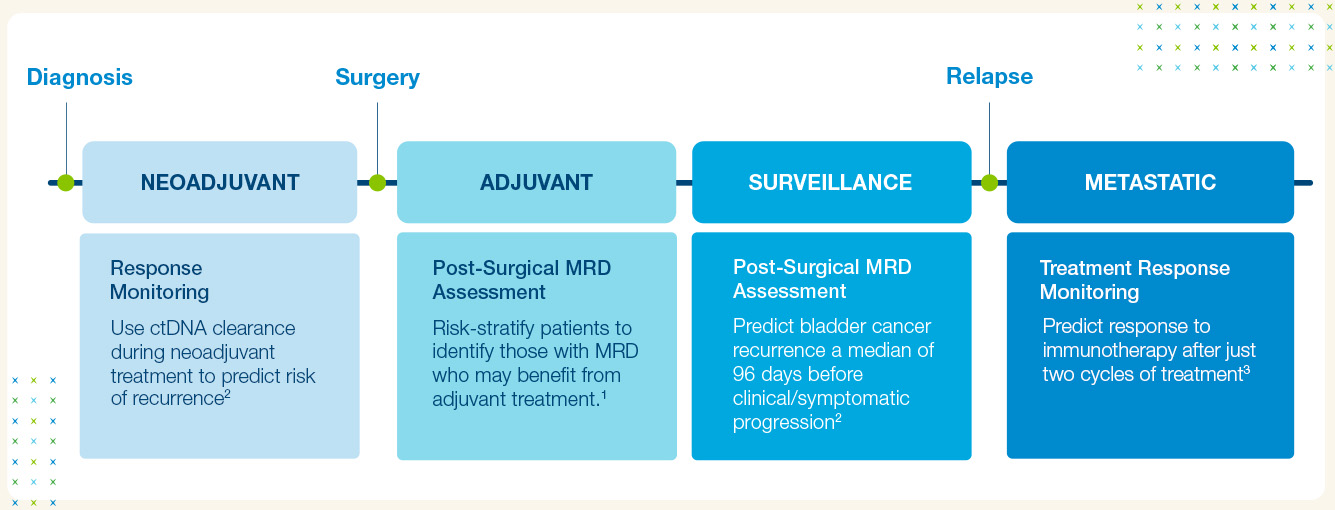
Signatera™ is Clinically Validated
- The Signatera™ MRD test is clinically validated across muscle invasive bladder cancer treatment settings.
Explore the peer-reviewed data.
-
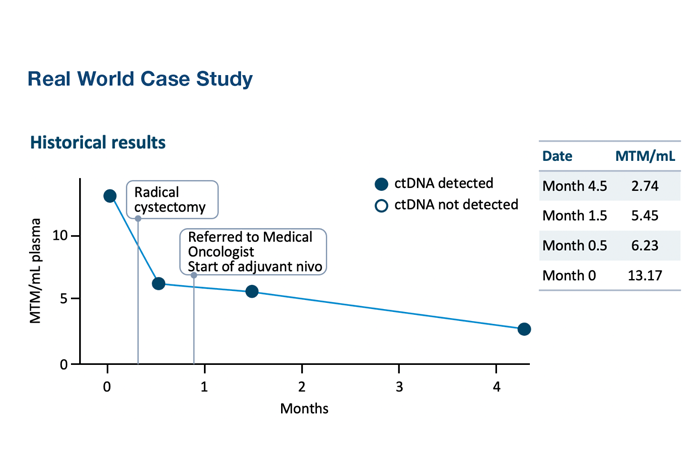
Aarhus University StudySignatera™ ctDNA status before neoadjuvant chemo, before cystectomy, and after cystectomy was highly prognostic of patient outcomes2
- Signatera™ ctDNA status was a stronger predictive factor for recurrence than lymph node status or pathologic staging with a lead time to clinical recurrence of up to 245 days (median=96 days)2
-
IMvigor010 Study
-
IMvigor011 Study
-
INSPIRE Study
Explore real-world cases
-
Post-Surgical MRD Assessment
Learn About Advances in ctDNA-Guided Treatment for Urothelial Carcinoma
In this webinar, Dr. Thomas Powles shares the findings of the IMvigor010 data analysis and potential applications for ctDNA testing in bladder cancer. Dr. Powles will discuss the utility of Signatera™ MRD testing for:
- Early detection of molecular relapse, potentially allowing clinicians to treat at lower levels of disease burden
- Predicting treatment response as early as cycle 3 of immunotherapy
- Future decision-making in the neoadjuvant setting
Discover the Published Data in Bladder Cancer
- PUBLICATIONS
- POSTERS
ABACUS: Neoadjuvant Atezolizumab in Cisplatin-ineligible Patients with Muscle-invasive Urothelial Cancer of the Bladder
ctDNA guiding adjuvant immunotherapy in urothelial carcinoma
The effect of surgical trauma on circulating free DNA levels in cancer patients - implications for studies of circulating tumor DNA
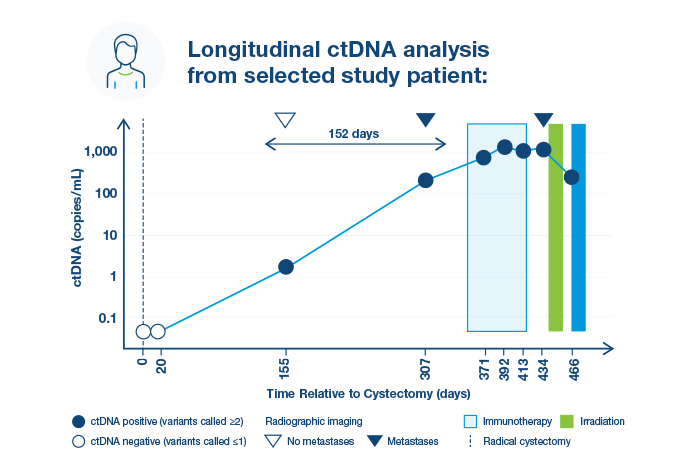
Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients with Urothelial Bladder Carcinoma
European Association of Urology (EAU) 2022
Gschwend JE, Assaf ZJ, Mariathasan S, et al. Overall survival by circulating tumor DNA status in patients with post-operative muscle-invasive urothelial carcinoma treated with atezolizumab: Update from IMvigor010. EAU, Amsterdam, Netherlands, July 1-4, 2022.
ASCO GU 2022
Powles T, Young A, Nimeiri H, et al. Molecular residual disease (MRD) detection with a tissue comprehensive genomic profiling (CGP)-informed personalized monitoring assay: An exploratory analysis of the IMvigor010 observation arm. ASCO Genitourinary Cancer Symposium, San Francisco, CA. Feb 17-19, 2022.
ESMO 2018
Birkenkamp-Demtröder K, Christensen E, Sethi H, et al. Sequencing of Plasma cfDNA from Patients with Locally Advanced Bladder Cancer for Surveillance and Therapeutic Efficacy Monitoring. Poster presented at: European Society for Medical Oncology; October 19-23, 2018; Munich, Germany.
AACR 2018
Birkenkamp-Demtröder K, Christensen E, Sethi H, et al. Sequencing of Plasma cfDNA from Patients with Locally Advanced Bladder Cancer for Surveillance and Therapeutic Efficacy Monitoring. Poster presented at: American Association for Cancer Research; April 14-18, 2018; Chicago, IL.
Medicare Coverage
- Stage II-IV and oligometastatic colorectal cancer (CRC) in the adjuvant and recurrence monitoring settings
- Muscle invasive bladder cancer (MIBC) in the adjuvant and recurrence monitoring settings
- Stage II-IV breast cancer in the neoadjuvant setting, regardless of subtype
- Stage IIb and higher breast cancer in the adjuvant and recurrence monitoring settings
- Stage I-III non-small cell lung cancer (NSCLC) in the surveillance setting
- Stage II-IV ovarian, fallopian tube, or primary peritoneal cancer in the adjuvant and recurrence monitoring settings
- For monitoring of response to immune-checkpoint inhibitor (ICI) therapy for patients with any solid tumor
Commercial Insurance
We will work with patients so that cost is not a barrier for testing.
We offer an affordable self-pay rate for those patients who do not wish to use insurance.
Learn More

Medicare Coverage
Signatera™ is covered by Medicare for immunotherapy treatment response monitoring across all solid tumor types and stages of cancer.

Bladder Cancer Brochure
Learn more about Signatera™ for bladder cancer

IMvigor010 Pub Summary
Learn more about the IMvigor010 trial and review Signatera™’s bladder cancer data in detail.
Is Signatera™ right for your bladder cancer patients?
References
1Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432-437. doi:10.1038/s41586-021-03642-9.
2Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol. 2019;37(18):1547-1557. doi:10.1200/JCO.18.02052.
3Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1:873-881. doi:10.1038/s43018-020-0096-5.
4Powles T, et al. European Urology. 2023; https://doi.org/10.1016/j.eururo.2023.06.007.
5Powles T, et al. Presented at EAU annual conference, 2024.