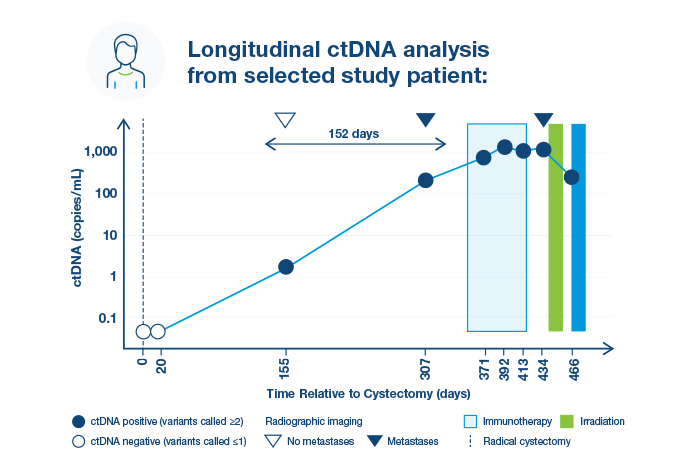
Signatera™ for Genitourinary Cancer (GU) Cancers
Signatera™ monitors ctDNA to provide molecular insights across GU cancers, helping clinicians:
- Identify patients at higher risk of recurrence across GU cancers
- Detect recurrence earlier than traditional standard of care tools
- Monitor treatment response to inform personalized care decisions
Signatera™ identifies MIBC patients more likely to benefit from adjuvant treatment1,2
Improved disease-free survival (DFS) and overall survival (OS) was observed in Signatera™-positive patients treated with adjuvant immunotherapy1,2
Signatera™ may help spare MIBC patients unnecessary treatment1,2
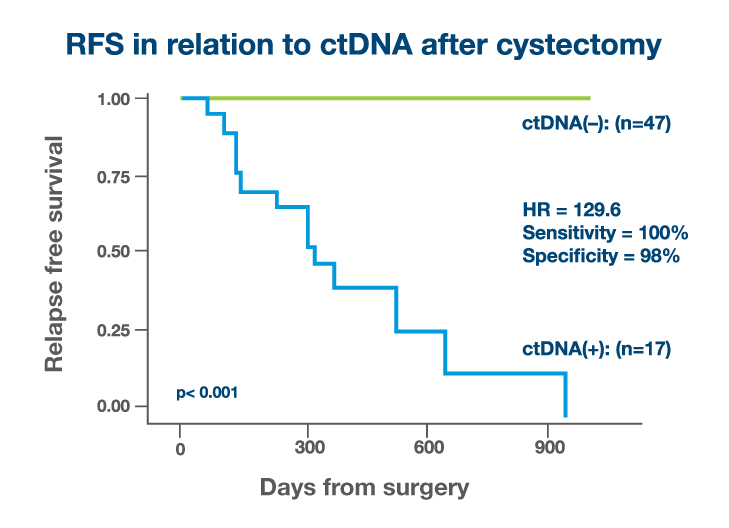
Serially Signatera™-negative patients had excellent survival outcomes without adjuvant therapy
Top-tier medical journals highlight the value of Signatera™-guided therapy in MIBC trials1,2
- IMvigor 011: published in The New England Journal of Medicine
- Checkmate 274: published in Annals of Oncology
Get Started
Personalized Signatera™ testing guides adjuvant therapy decisions in MIBC1
The Phase 3 IMvigor011 trial demonstrated that Signatera™-positive patients treated with adjuvant atezolizumab achieved statistically significant and clinically meaningful improvements in survival outcomes:
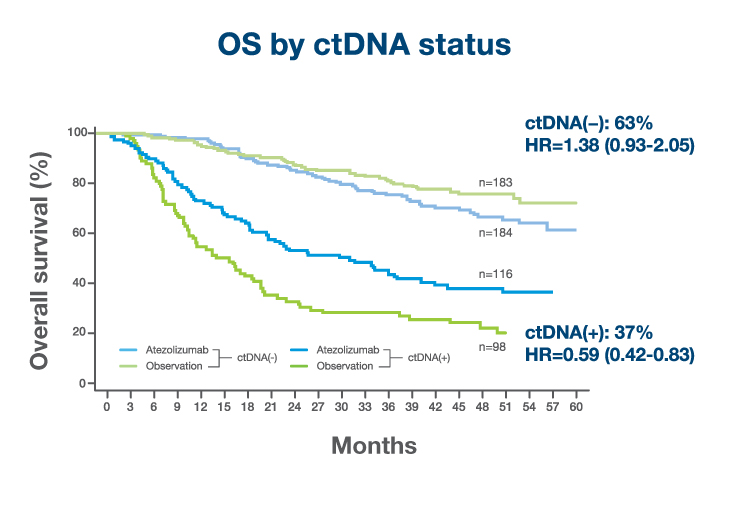
OS in Signatera™-positive patients
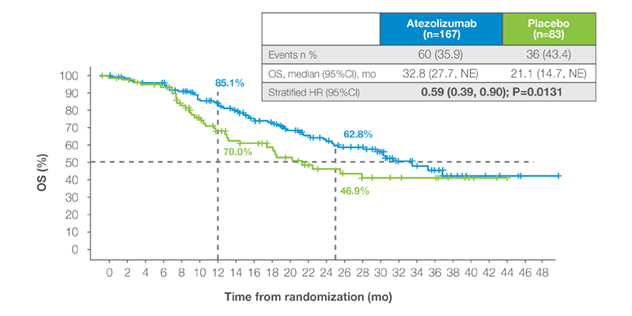
- 41% reduction in risk of recurrence or death (OS HR 0.59)1
- Overall survival benefit confirming the clinical value of Signatera™-guided treatment1
OS in Signatera™-negative patients
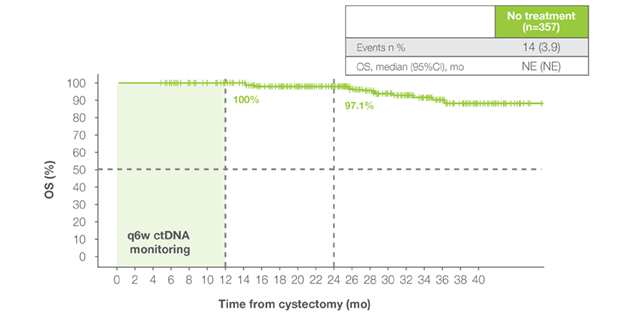
- Signatera™-negative patients maintained excellent outcomes with surveillance alone (1-year OS 96%; 2-year DFS 88%)1
- Establishes the first prospective, randomized evidence that personalized, serial MRD testing can expand the adjuvant window in MIBC1
Signatera™ is Clinically Validated
- The Signatera™ MRD test is validated in two landmark Phase 3 adjuvant trials in MIBC, demonstrating how personalized MRD testing can safely expand the adjuvant window.1,2
Explore the peer-reviewed data.
-
IMvigor011 Study
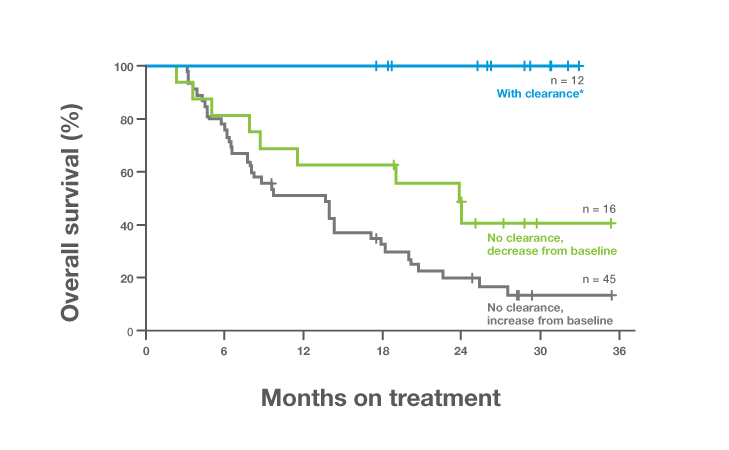
DFS in Signatera™-negative patients
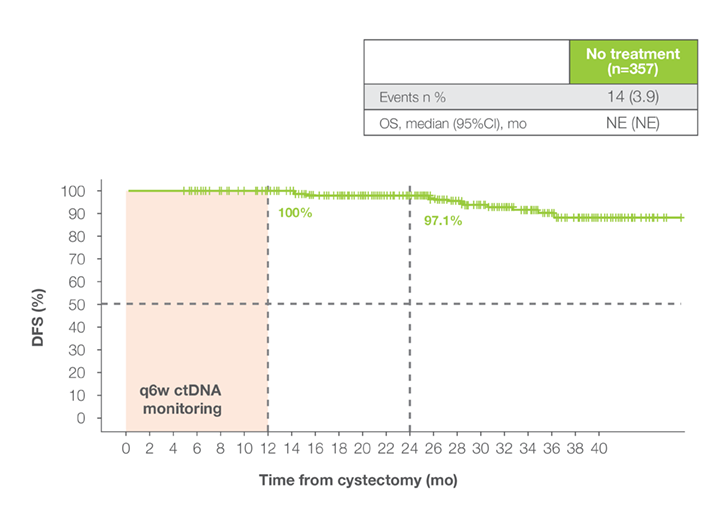 Patients who remained serially Signatera™-negative post-cystectomy achieved exceptional outcomes:
Patients who remained serially Signatera™-negative post-cystectomy achieved exceptional outcomes:- OS rates of 100% at 12 months and 98% at 18 months1
- DFS rates of 92% at 12 months and 88% at 18 months1
- These results support safe surveillance for Signatera™-negative patients, minimizing overtreatment and toxicity1
-
IMvigor010 Study
-
INSPIRE Study
-
Aarhus University Study
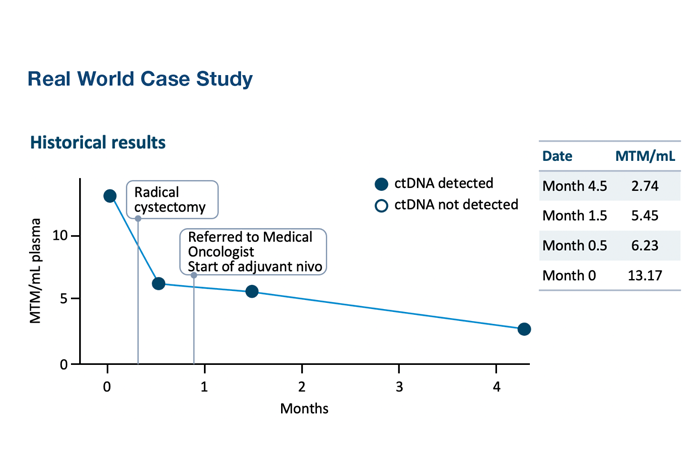
Explore real-world cases
-
Post-Surgical MRD Assessment
Get Actionable Insights Across the Genitourinary (GU) Cancer Care Journey
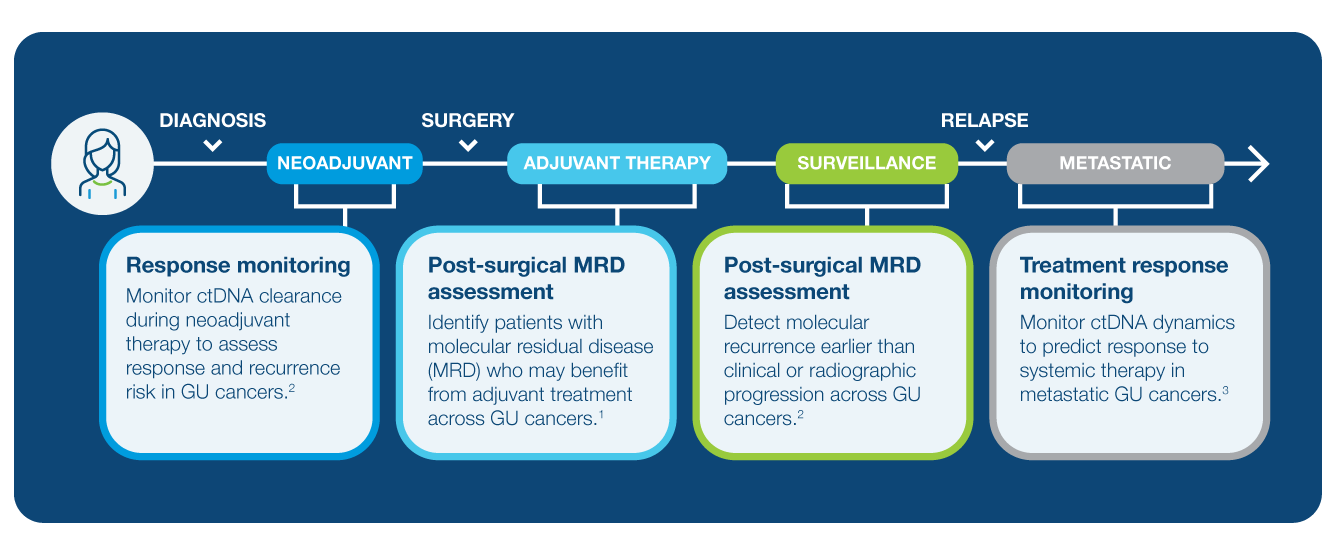
Discover the Published Data in Bladder Cancer
- PUBLICATIONS
- POSTERS
IMvigor011: ctDNA-Guided Adjuvant Atezolizumab in Muscle-Invasive Bladder Cancer
CheckMate 274: Adjuvant Nivolumab Versus Placebo and Exploratory Signatera™ ctDNA Analysis
The effect of surgical trauma on circulating free DNA levels in cancer patients - implications for studies of circulating tumor DNA
Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients with Urothelial Bladder Carcinoma
European Association of Urology (EAU) 2022
Gschwend JE, Assaf ZJ, Mariathasan S, et al. Overall survival by circulating tumor DNA status in patients with post-operative muscle-invasive urothelial carcinoma treated with atezolizumab: Update from IMvigor010. EAU, Amsterdam, Netherlands, July 1-4, 2022.
ASCO GU 2022
Powles T, Young A, Nimeiri H, et al. Molecular residual disease (MRD) detection with a tissue comprehensive genomic profiling (CGP)-informed personalized monitoring assay: An exploratory analysis of the IMvigor010 observation arm. ASCO Genitourinary Cancer Symposium, San Francisco, CA. Feb 17-19, 2022.
ESMO 2018
Birkenkamp-Demtröder K, Christensen E, Sethi H, et al. Sequencing of Plasma cfDNA from Patients with Locally Advanced Bladder Cancer for Surveillance and Therapeutic Efficacy Monitoring. Poster presented at: European Society for Medical Oncology; October 19-23, 2018; Munich, Germany.
AACR 2018
Birkenkamp-Demtröder K, Christensen E, Sethi H, et al. Sequencing of Plasma cfDNA from Patients with Locally Advanced Bladder Cancer for Surveillance and Therapeutic Efficacy Monitoring. Poster presented at: American Association for Cancer Research; April 14-18, 2018; Chicago, IL.
Testicular Cancer
Signatera™’s clinical validation in testicular cancer ctDNA studies
A recent series of prospective studies evaluated whether Signatera™ could improve recurrence detection and assess treatment response in patients with testicular cancer.
- In patients with stage I testicular cancer, tumor-informed ctDNA demonstrated a specificity of 96.3% and NPV of 86.7% compared with imaging for detecting recurrence; undetectable serial ctDNA results may help reduce unnecessary imaging surveillance.6
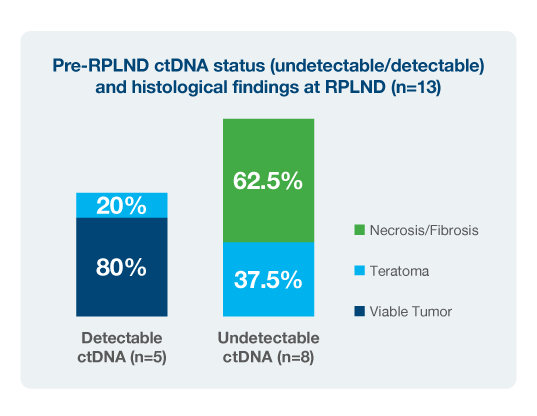
- In the RPLND setting, detectable ctDNA in the post-operative MRD window was strongly associated with progression: all patients with detectable ctDNA relapsed, whereas none with undetectable ctDNA experienced recurrence.7
Detect recurrence early and monitor treatment response with Signatera™
Signatera™ detected testicular cancer recurrence with 96% specificity and 87% NPV compared to imaging in stage I disease, and predicted progression with 100% accuracy in the post-RPLND MRD setting
- In patients with stage I testicular cancer, ctDNA demonstrated 96.3% specificity and 86.7% NPV, suggesting serial negative results could allow safe reduction of imaging burden.9
- Among patients who underwent RPLND, all with detectable ctDNA in the post-operative MRD window progressed, while none with undetectable ctDNA recurred.9
- Patients with persistently negative ctDNA after treatment remained disease-free during surveillance, supporting its role in guiding escalation or de-escalation of therapy.
Medicare Coverage
- Stage II-IV and oligometastatic colorectal cancer (CRC) in the adjuvant and recurrence monitoring settings
- Muscle invasive bladder cancer (MIBC) in the adjuvant and recurrence monitoring settings
- Stage II-IV breast cancer in the neoadjuvant setting, regardless of subtype
- Stage IIb and higher breast cancer in the adjuvant and recurrence monitoring settings
- Stage I-III non-small cell lung cancer (NSCLC) in the surveillance setting
- Stage II-IV ovarian, fallopian tube, or primary peritoneal cancer in the adjuvant and recurrence monitoring settings
- For monitoring of response to immune-checkpoint inhibitor (ICI) therapy for patients with any solid tumor
Commercial Insurance
We will work with patients so that cost is not a barrier for testing.
We offer an affordable self-pay rate for those patients who do not wish to use insurance.
Learn More

Medicare Coverage
Signatera™ is covered by Medicare for immunotherapy treatment response monitoring across all solid tumor types and stages of cancer.

Bladder Cancer Brochure
Learn more about Signatera™ for bladder cancer

IMvigor010 Pub Summary
Learn more about the IMvigor010 trial and review Signatera™’s bladder cancer data in detail.
Is Signatera™ for GU cancer right for your patients?
References
1Powles T, Kann AG, Castellano D, et al. ctDNA-Guided Adjuvant Atezolizumab in Muscle-Invasive Bladder Cancer.New England Journal of Medicine. Published online October 20, 2025. doi:10.1056/NEJMoa2508652.
2Galsky MD, Gschwend JE, Milowsky MI, et al. Adjuvant Nivolumab Versus Placebo for High-Risk Muscle-Invasive Urothelial Carcinoma: 5-Year Efficacy and ctDNA Results from CheckMate 274. Annals of Oncology. Published online October 17, 2025. doi:10.1016/j.annonc.2025.09.139.
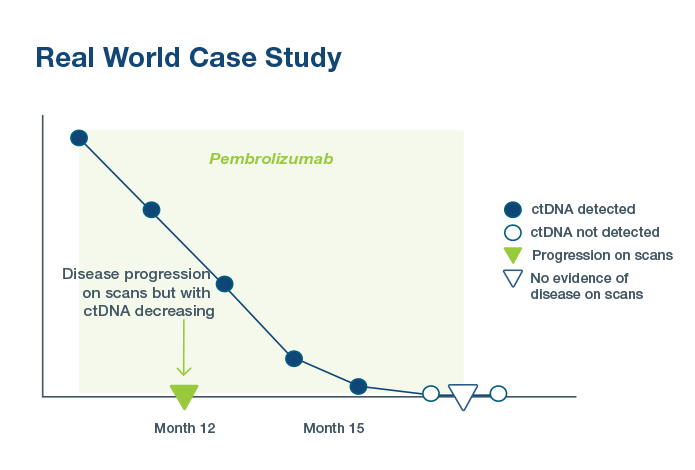
3Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1:873-881. doi:10.1038/s43018-020-0096-5.
4Powles T, et al. European Urology. 2023; https://doi.org/10.1016/j.eururo.2023.06.007.
5Powles T, et al. Presented at EAU annual conference, 2024.
6American Cancer Society. Key statistics for testicular cancer. 2025. Available at: https://www.cancer.org/cancer/types/testicular-cancer/about/key-statistics.html
7Reuben Ben-David, et al. Utility of tumor-informed circulating tumor DNA (ctDNA) in patients undergoing retroperitoneal lymph node dissection for testicular cancer. Icahn School of Medicine at Mount Sinai. Poster presented in 2023.
8Parth Thakker, et al. Circulating tumor DNA as a prognostic biomarker to predict retroperitoneal histology in patients undergoing retroperitoneal lymph node dissection. Indiana University. Poster presented at ASCO GU, 2025.
9Reuben Ben-David, et al. Comparative performance of tumor-informed circulating tumor DNA and imaging studies in monitoring patients with Stage I testicular cancer. Icahn School of Medicine at Mount Sinai. Poster presented at ASCO GU, 2024.