Be Proactive with Prospera™ to Surveil Your New Kidney
More informed, better protection
You and your provider understand the importance of preserving the function of a newly transplanted kidney. Achieving this goal could mean avoiding both dialysis and returning to the transplant list.
Early, informed treatment of underlying disease and patient adherence to medications are essential to preserving kidney function.

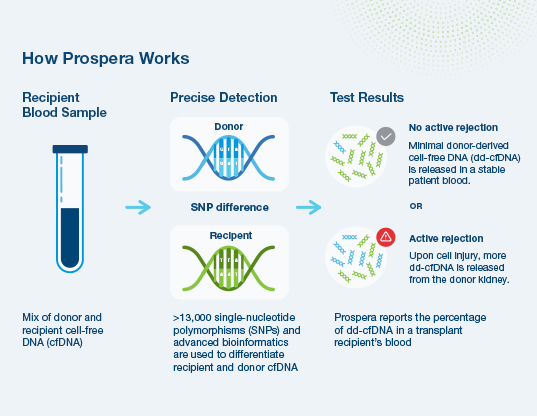
How Does It Work?
From a single blood draw, Prospera™ measures the amount of donor DNA from your transplanted kidney in your blood. This helps your care team assess all types of rejection more precisely than available standard assessment tools.1
What do the Prospera™ Results Mean to Me?
The Prospera™ result represents the percent of cell-free DNA in your blood that originates from the donated kidney to determine whether or not you may be experiencing active rejection. It may also indicate other types of kidney injury you are experiencing.
Like your other regular monitoring tests, Prospera™ is recommended for periodic use over time as directed by your doctor.
A way to track your cell-free DNA over time
- Establishing a baseline tells you and your care team the “normal state” of your new kidney. You can measure new results against this baseline.
- Following your levels in the future reveals your new kidney’s health
If a Prospera result is above 1%
This may mean that active rejection is occurring2. Terms used to describe the various types of active rejection include:
- Antibody-mediated rejection
- T cell-mediated rejection
- Mixed rejection
To confirm a rejection or the type of rejection, you should consult your doctor.
If a Prospera™ result is in the normal range
This may mean that your kidney is stable1.
ProsperaLink Program: Your Support Team
We offer complete support through our Prospera™ Link Program:
- Always by your side: Natera’s care team will guide you through the process of using Prospera™ and check-in with you at every milestone
- Flexible for your convenience: Our team coordinates blood draws around your schedule — at a certified laboratory near you or by a blood draw specialist who can come to you
- Transparent & accessible: Our proactive billing outreach and flexible payment plans, including assistance for hardship, help ensure no significant financial hardship in accessing Prospera™. Natera welcomes all insurances. Prospera™ is covered by Medical for assessing potential kidney transplant rejection.

Sign up to find out if you’re eligible for Prospera™
References
1Sigdel TK et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med. 2019;8(1):19.



