Liquid biopsy vs. Signatera ctDNA cancer monitoring test

A deep dive into liquid biopsies vs. SignateraTM ctDNA cancer monitoring test
With newer cancer tests now available, it’s not surprising that cancer patients, survivors and their families are seeking information about every option to help them make the best decisions. In this blog post, we focus on Signatera and liquid biopsies, explaining what these tests involve, how they are performed, when they are used, and how they differ from each other.
What is a liquid biopsy?
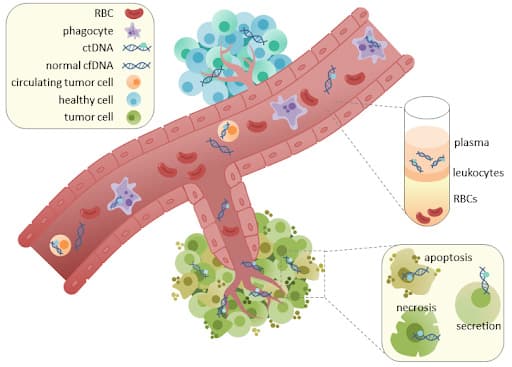
By Racheljunewong – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=56676758
What is Signatera ctDNA cancer monitoring test?

- after surgery, to detect the presence of any residual disease
- during treatment, to evaluate treatment response
- after treatment, to monitor for early recurrence
The key differences between Signatera and liquid biopsies
- Both blood and tissue vs. blood only: Unlike liquid biopsies, Signatera uses both your blood and tumor tissue to create a custom-built test that tracks your unique tumor DNA mutations. On the other hand, liquid biopsy may be useful for patients where tumor tissue is not available or ideal to extract.
- Tumor-informed vs. tumor-naive: Signatera selects and tracks tumor mutations that are specific to your individual tumor and that would be present in all future cancer cells. This results in highly accurate cancer monitoring over time. Liquid biopsies use a tumor-naive approach based on detection of a predetermined panel of genetic alterations, making them less sensitive and specific.
- Specific context vs. wider range of applications: Signatera is used in a specific context of MRD, cancer monitoring and treatment response, where it’s been proven to deliver exceptional accuracy. Liquid biopsies have been primarily used to identify actionable markers for therapy selection decisions or to predict treatment response.
Additional Resources
Video: About Signatera and ctDNA
References
1Su Y-H. Liquid biopsy: an old concept with a new twist. Genetic Engineering & Biotechnology News. April 18, 2019. Accessed September 23, 2020. https://www.genengnews.com/insights/liquid-biopsy-an-old-concept-with-a-new-twist/
2Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7(30):48832-48841. doi: 10.18632/oncotarget.9453
3Liquid biopsy. National Cancer Institute. Accessed September 23, 2020. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/liquid-biopsy
4Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018;19(10):2877. doi: 10.3390/ijms19102877
5Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13:34. doi: 10.1186/s40246-019-0220-8
6Mattox AK, Bettegowda C, Zhou S, Papadopoulos N, Kinzler KW, Vogelstein B. Applications of liquid biopsies for cancer. Sci Transl Med. 2019;11(507) doi: 10.1126/scitranslmed.aay1984
