Empower now offers Tyrer-Cuzick on reports
Personalized breast cancer risk assessment
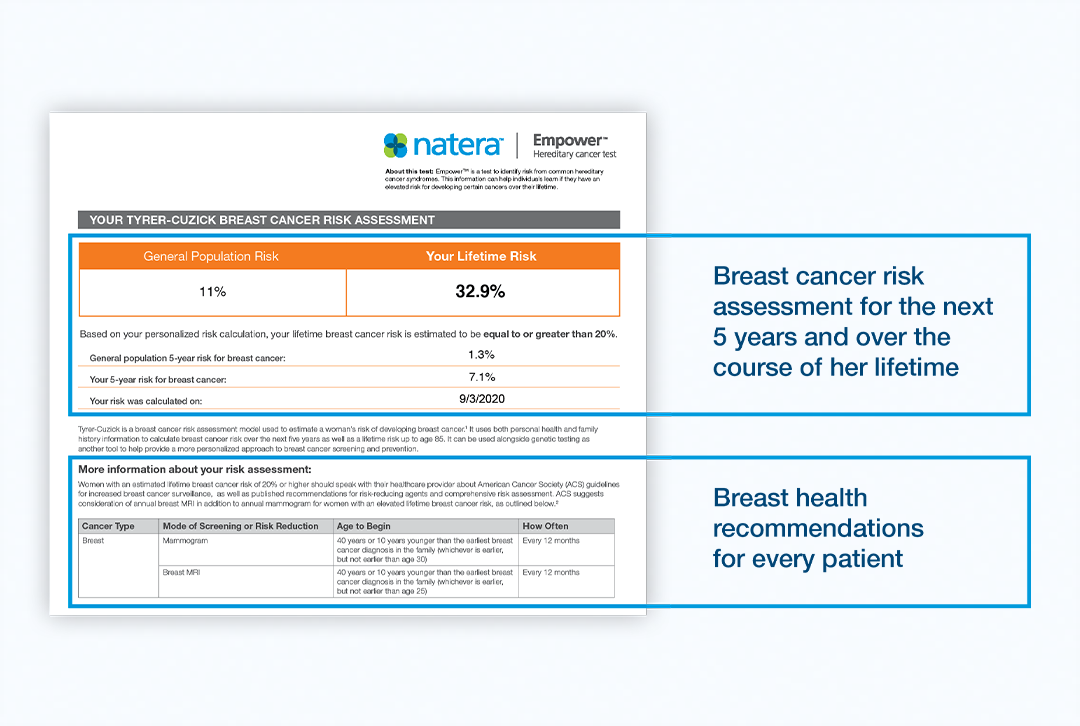
The Empower Hereditary Cancer Test now supplements test results with a personalized breast cancer risk assessment. The new addition uses the Tyrer-Cuzick risk model to calculate your patient’s individual risk of developing breast cancer. Tyrer-Cuzick integrates a variety of inputs from the patient, including family and personal history, to calculate their 5-year and lifetime risks of breast cancer.
Tyrer-Cuzick reports this risk as a percentage, indicating their probability of developing breast cancer. This information has value, as patients with ≥20% remaining lifetime breast cancer risk can receive more sensitive screenings like breast MRI covered by their insurance. The American Cancer Society (ACS), the National Comprehensive Cancer Network (NCCN), and the American Congress of Obstetricians and Gynecologists recommend additional breast MRI screening for high-risk patients.1,2
With this additional feature, you can personalize your patient’s medical management plan.
Choosing your risk assessment
Tyrer-Cuzick can further refine a woman’s risk for breast cancer based on her family history and other lifestyle factors. The Tyrer-Cuzick model leverages other inputs from the patient – family history of cancer, breast density measurements, personal history of breast biopsy, and lifetime exposure to estrogen. These factors have been shown to increase a woman’s risk of developing breast cancer in addition to genetic risk factors. All women under age 85 are eligible, provided they have not been diagnosed with breast cancer or have tested positive for a breast cancer-related gene mutation.
Tyrer-Cuzick is not a polygenic risk score (PRS). While PRSs uses genetic data (namely, single nucleotide polymorphisms) from genome-wide association studies (GWAS) to calculate a patient’s relative risk of developing breast cancer, the NCCN does not recommend PRS for clinical management of breast cancer.

Individualized risk assessment made easy
Natera makes it easy for you to collect inputs from your patients on screening forms, test request forms, or with Natera’s Educational Virtual Assistant — NEVA for short. NEVA is a HIPAA-compliant assistant that allows your patients to enter their own information for a Tyrer-Cuzick analysis on their phone or computer 24/7.
Natera is committed to making high-quality hereditary cancer testing accessible for you and your patients. Natera billing practices value price transparency and accessibility for anyone who can benefit from hereditary cancer risk assessment. Learn more about Natera's billing practices here. Contact us to learn more about this exciting addition to the Empower Hereditary Cancer Test.
References
- Himes DO, Root AE, Gammon A, Luthy KE. Breast Cancer Risk Assessment: Calculating Lifetime Risk Using the Tyrer-Cuzick Model. The Journal for Nurse Practitioners. 2016 Oct. 12 (9): 581-592.
- American Cancer Society Recommendations for the Early Detection of Breast Cancer. Nov 2020. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
- NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment Breast, Ovarian, and Pancreatic V1.2021
