NKF and KDIGO recommend genetic testing for kidney disease
Learn MoreNKF and KDIGO recommend genetic testing for kidney disease
Learn MoreWhat are the benefits of genetic testing for kidney disease?
Provides valuable information for disease management
- Identify the cause of disease and help predict its progression
- Impact treatment path by using test results to inform more tailored interventions
- Educate family members who may also be at risk for kidney disease
- Enroll in appropriate clinical trials that may provide advanced treatments and interventions

Why choose Renasight?
Comprehensive. Convenient. Accessible.
- Comprehensive: Leverages next generation sequencing to analyze 397 genes that were selected by genetic experts to provide clinically actionable information.
- Convenient: Remote testing services including virtual ordering for providers and at home sample collection for patients (saliva or mobile blood draw). Complimentary information sessions with board-certified genetic counselors.
- Accessible: Affordable with limited to no patient responsibility for most patients. All insurance plans accepted and affordable testing is available through a variety of payment methods.
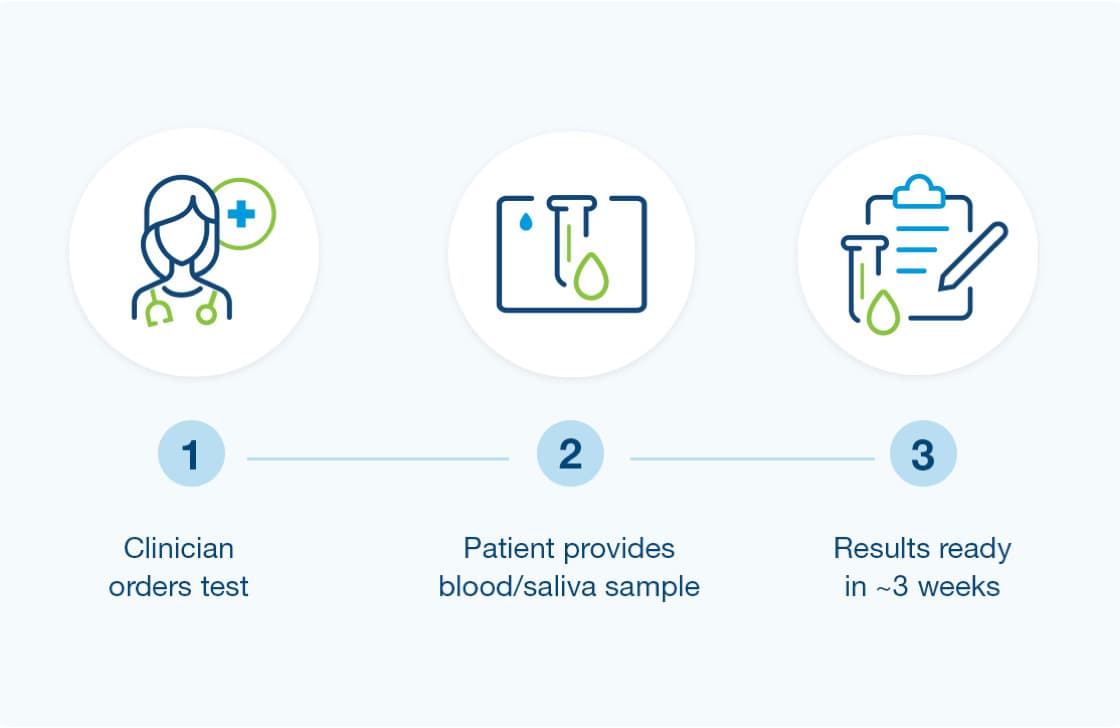
How to get started
- Provider orders the Renasight test
- Patient provides a blood or saliva sample (from clinic or home)
- Results are available for review in about 3 weeks.