Better biomarkers are needed to help inform sarcoma patient management
- Sarcoma is an aggressive cancer that is diagnosed in ~17,000 patients annually across over 70 distinct histological subtypes, with up to 40% of patients experiencing progression to metastatic disease.1-3
- Due to its heterogeneity, Sarcoma recurrence detection and treatment response monitoring can be challenging with standard imaging techniques and clinical assessment.
How Signatera™ Works: a personalized and tumor informed approach to MRD surveillance
Personalized, tumor-informed assay
One-time, primary tissue sample and matched normal sample is required for whole exome or whole genome sequencing and personalized test design.
Ultrasensitive ctDNA detection
Signatera™ is designed to detect ctDNA of somatic and truncal variants to optimize sensitivity. Tumor-informed method enables filtering of CHIP mutations to decrease false positive rates.
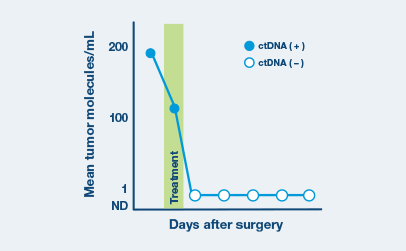
Optimized for longitudinal monitoring
Once the patient’s personalized test has been designed, only a blood sample is needed each subsequent time.
Signatera™’s clinical validation in the largest sarcoma ctDNA study to-date
A recent study conducted by Stanford University set out to determine if Signatera™ could help better detect recurrences and measure response to treatment4
- The study assessed the correlation of Signatera™ results with imaging, stratified by sarcoma subtype, and followed patients through treatment, progression, and surveillance.
- >2,000 samples were collected from 210 sarcoma patients across 5 tumor sites and >15 subtypes.
Detect recurrence early and monitor treatment response with Signatera™
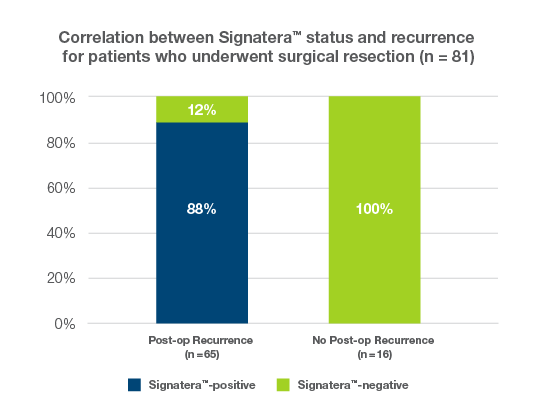
Signatera™ detected sarcoma recurrence with 89% sensitivity and 100% specificity across tumor sites and subtypes
- In leiomyosarcoma, the most common subtype in the cohort, sensitivity and specificity were 93% and 100% respectively, demonstrating a 95% ctDNA correlation.
- For leiomyosarcoma patients who experienced progression, ctDNA dynamics during subsequent therapy were highly correlated with treatment response (90%).
- All 16 patients who were serially Signatera™-negative remained recurrence free with a median follow-up of 22 months.
Not all MRD assays are created equal
Considerations when choosing patient monitoring tools:
To get started, send in your Signatera™ requisition form, sign in to the online portal or talk to your sales representative.
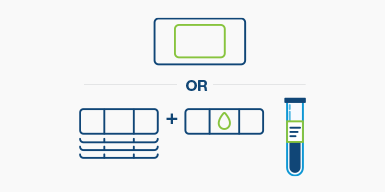
Step 1
Send tumor block or slides to prepare the personalized tumor-informed assay

Step 2
Complete information on tube labels
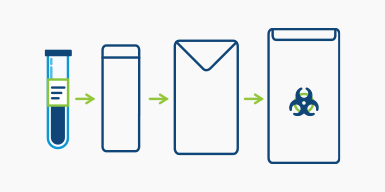
Step 3
Place filled and labeled tubes into the absorbent sleeve and into the metallic envelope. Place the metallic envelope into the biohazard bag.
Is Signatera™ for Sarcoma right for your patients?
We’re here to help you find out
1Moffitt Cancer Center. Moffitt Cancer Center. What are the different types of sarcoma? https://www.moffitt.org/cancers/sarcoma/faqs/what-are-the-different-types-of-sarcoma/. Accessed March 31, 2025.
2Sarcoma Alliance. (n.d.). What is sarcoma? Sarcoma Alliance. Retrieved April 28, 2025, from https://sarcomaalliance.org/what-is-sarcoma/.
3Spalato-Ceruso M, Ghazzi NE, Italiano A. New strategies in soft tissue sarcoma treatment. Journal of Hematology & Oncology. 2024;17:76. https://doi.org/10.1186/s13045-024-01580-3
4Sun B, et al. Using circulating tumor DNA to monitor sarcoma treatment and recurrence. Presented at the 2025 SSO Annual Meeting.