Natera Acquires Foresight Diagnostics, Accelerating Leadership in MRD Detection
Learn MoreNatera Acquires Foresight Diagnostics, Accelerating Leadership in MRD Detection Learn More
When to use Signatera™ ctDNA-MRD test?
Newly Diagnosed Setting
- Assess response to first line therapy to help inform next steps
- Guidelines recommend ctDNA-MRD testing PET-positive DLBCL patients after frontline therapy
Relapsed/Refractory Setting
- Real-time risk assessment for patients with a high rate of relapse
- Assess the patient’s response to CAR-T cell therapy or autologous stem cell transplant to determine if further treatment is necessary
Surveillance Setting
- Detect recurrence with greater sensitivity than current standard of care tools
- Signatera™ is meant to be used serially to detect relapse earlier
How Signatera™ Works: a personalized and tumor informed approach to MRD surveillance
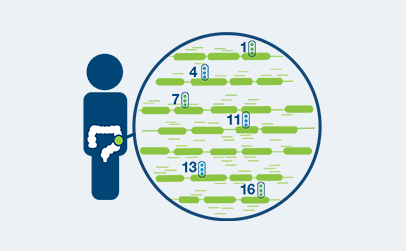
Personalized, tumor-informed assay
One-time, primary tissue sample and matched normal sample is required for whole exome or whole genome sequencing and personalized test design.
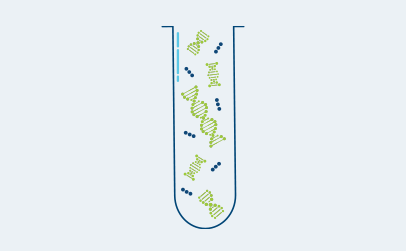
Ultrasensitive ctDNA detection
Signatera™ is designed to detect ctDNA of somatic and truncal variants to optimize sensitivity. Tumor-informed method enables filtering of CHIP mutations to decrease false positive rates.
Optimized for longitudinal monitoring
Once the patient’s personalized test has been designed, only a blood sample is needed each subsequent time.
Signatera™ MRD testing to improve patient management in lymphoma
- Despite high initial response rates to first-line therapy (R-CHOP), up to 30–40% of diffuse large b-cell patients will relapse
- Managing relapse, especially in older or frail patients who are not candidates for intensive therapies, is a growing challenge
- Predicting relapse early can make a difference in outcomes but current tools fall short at detecting disease recurrence
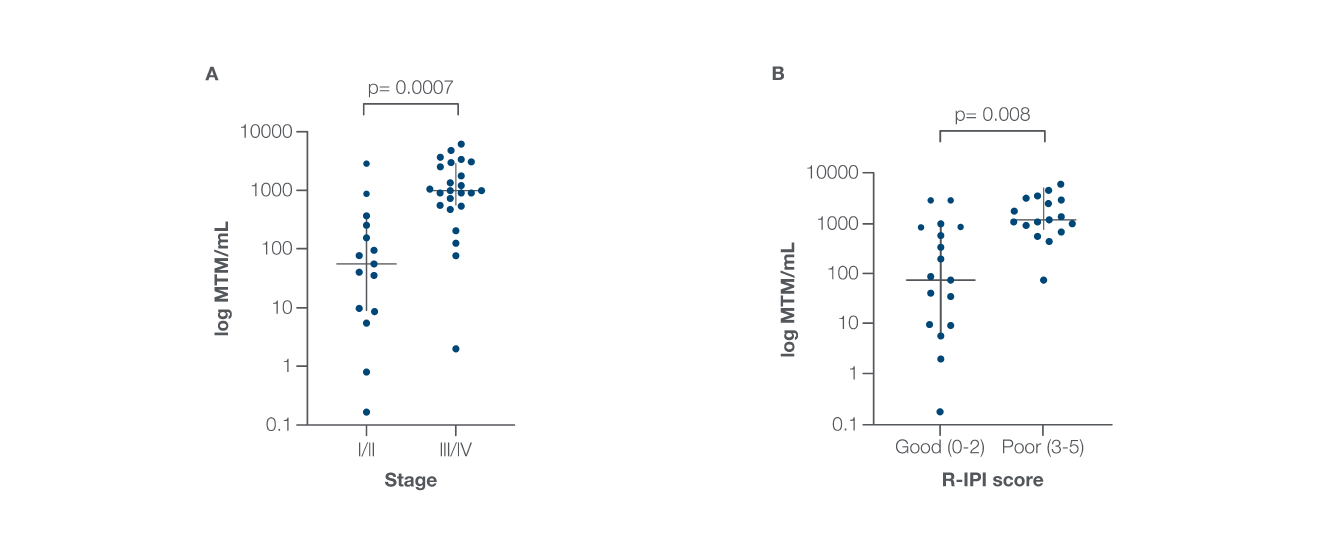
Study Findings: Baseline ctDNA levels correlated with stage and R-IPI score
- 95% ctDNA detection at baseline for patients with pre-treatment time points available
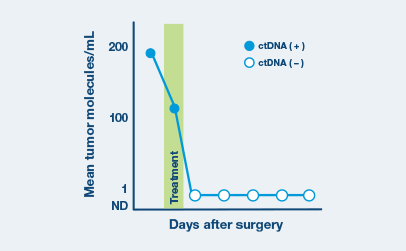
- Clearance of ctDNA during treatment strongly correlated with improved Event-Free Survival (EFS) and Overall Survival (OS) and early identification of responders and non-responders.
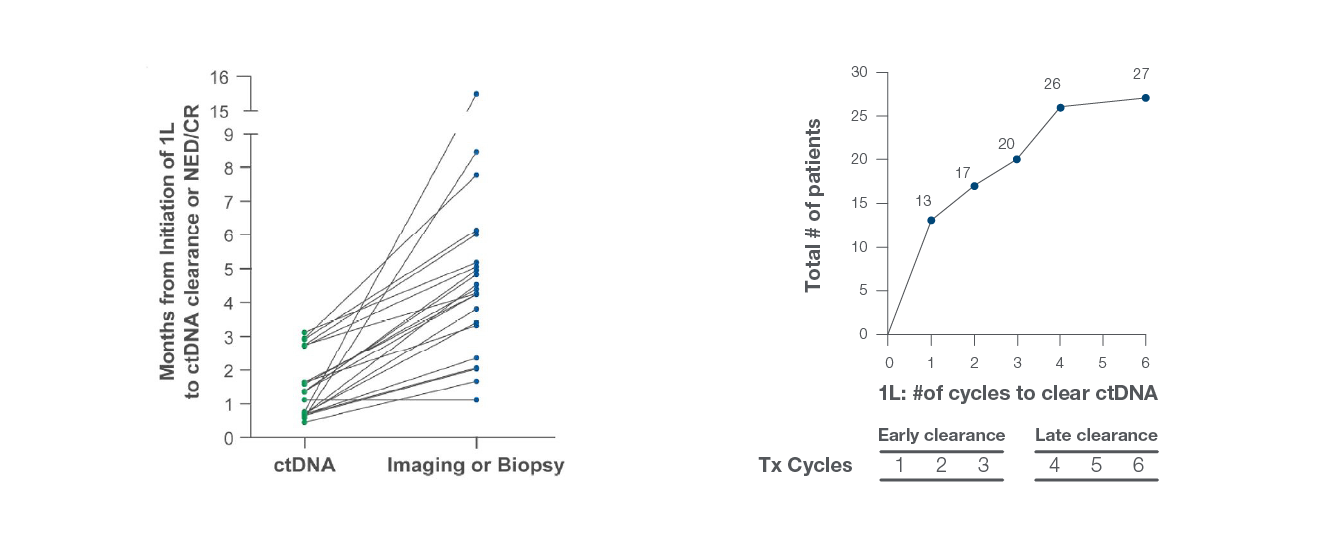
ctDNA clearance at any point during frontline therapy was found to be prognostic for improved survival outcomes
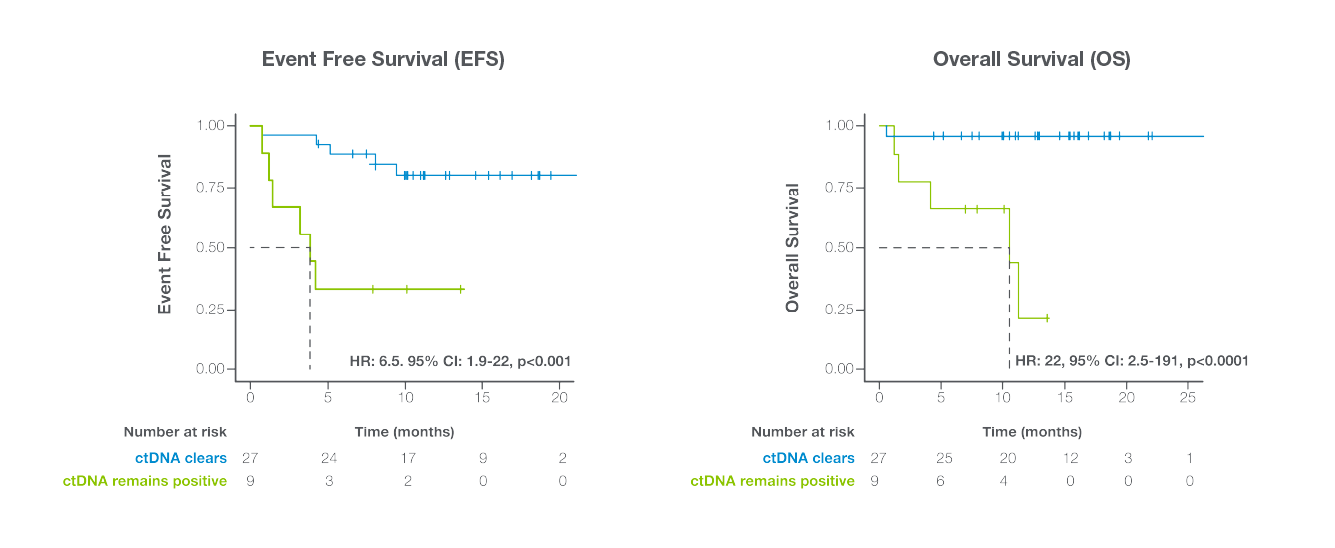
Signatera™ has demonstrated high clinical sensitivity (88.8%) and specificity (94.1%) in real-world settings for early, personalized insights into treatment efficacy, relapse risk, and residual disease.
ctDNA clearance at any point during first-line therapy was found to be predictive of improved response rate and outcomes
Not all MRD assays are created equal
Considerations when choosing patient monitoring tools:
To get started, send in your Signatera™ requisition form, sign in to the online portal or talk to your sales representative.
Step 1
Send tumor block or slides to prepare the personalized tumor-informed assay

Step 2
Complete information on tube labels
Step 3
Place filled and labeled tubes into the absorbent sleeve and into the metallic envelope. Place the metallic envelope into the biohazard bag.
Is Signatera™ in lymphoma right for your patients?
We’re here to help you find out
1Narkhede M, Tomassetti S, Iqbal M. Tumor-informed ctDNA assessment as a valuable prognostic and predictive biomarker in diffuse large B-cell lymphoma. Frontiers in Oncology. July 28, 2024; Section: Hematologic Malignancies. Volume 14 – 2024.