Power Clinical Decisions in Gynecologic Cancer
Understand Risk
after completion of adjuvant/definitive therapy, ctDNA was detectable in
23%
of patients, all of whom experienced disease progression (HR: 17.6 95%CI: 3.2-97.4, p<0.001)1
Detect Recurrence Earlier
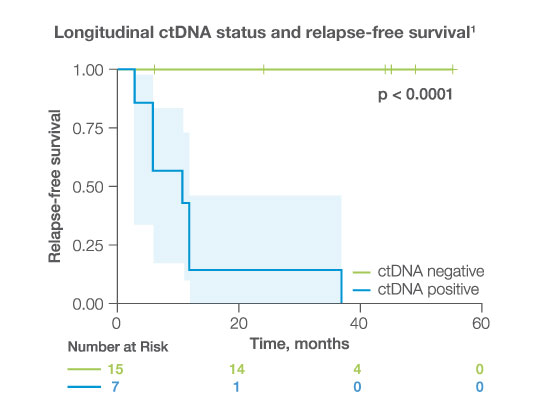
Longitudinally, ctDNA was detected with
100%
sensitivity and specificity while CA-125 had much lower sensitivity (43%) and specificity (78%)1,2
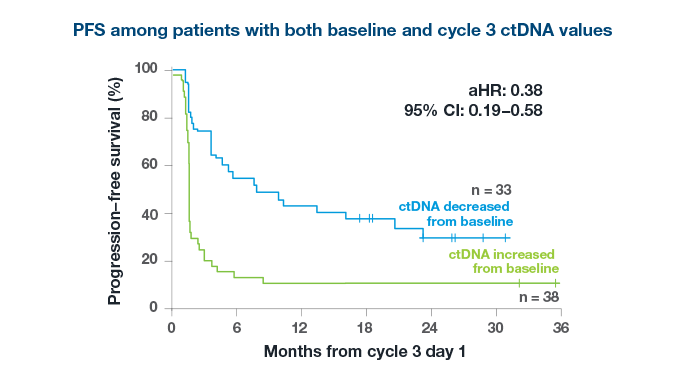
Predict Treatment Response
After just 2 cycles of immunotherapy
98%
of patients with an increase in ctDNA did not derive an objective response to treatment3*
Actionable Molecular Insights Across the Patient Journey
Signatera™ enables early detection of molecular residual disease (MRD) to help you manage your patients with gynecologic cancers.
Discover the Latest Data in Ovarian Cancer
ctDNA positivity post-ACT and pre-PARPi therapy was associated with significantly worse PFS
Median PFS for ctDNA-positive patients was 5.2 months, whereas none of the ctDNA-negative patients had progressed at last known follow-up

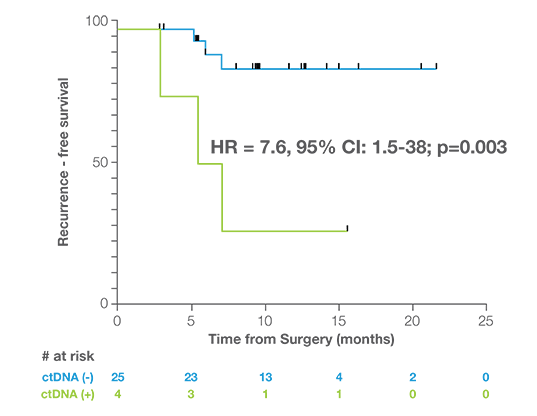
Discover the Latest Data in Uterine Cancer
Post Surgery MRD Assessment
Patients who were ctDNA positive within 2.5 months of surgery were 7.6x more likely to experience recurrence than ctDNA negative patients.
More Data in Ovarian Cancer
- Signatera™ is clinically validated cross multiple indications.
-
Post-Adjuvant MRD assessment
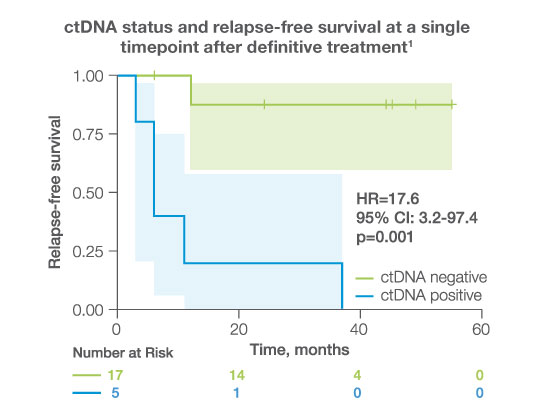
- After completion of adjuvant/definitive therapy, ctDNA was detectable in 23% of patients, all of whom experienced disease progression.1
-
Surveillance/Maintenance
-
Treatment Response Monitoring

Monitor Your Patients With Confidence
As part of her ovarian cancer monitoring plan, Anne received Signatera™ testing and CA-125 at regular intervals.
A positive Signatera™ result alerted her oncologist to order a scan, which caught a recurrence early. Now, Anne is receiving Signatera™ to monitor response to her maintenance therapy. Since starting therapy, Anne’s Signatera™ results have been negative, so she can feel more confident about her treatment plan.
Tailor Gynecologic Cancer Treatment With Natera’s Portfolio of Genomic Tests
| Signatera™ | Highly sensitive and perosnalized tumor-informed test for molecular residual disease (MRD) detection |
|---|---|
| Altera™ | Comprehensive genomic profiling for clinically relevant biomarkers that may help guide treatment selection (including MSI, BRCA1/2, HR genes, MMR genes, TMB, BRAF, RET, and NTRK), with no additional tumor sample needed. |
| Empower™ | Germline genetic test for commonly screened genes in gynecologic cancers (eg BRCA 1/2, MMR genes) to inform therapeutic decisions. |
More Clinician Resources
Signatera™ in Gynecologic Cancers
Read about the clinical utility of the Signatera™ test and data for MRD assessment, recurrence monitoring, and IO treatment response monitoring.

IO Monitoring Brochure
Learn how Signatera™ can assess immunotherapy response as early as 6 weeks into treatment for patients with ovarian, cervical, endometrial, and other cancers.2
Is Signatera™ for gynecologic cancer right for your patients?
References
1Hou et al. Gynecol Oncol. 2022; 167:334-341.
2Chapman et al. Poster presented at 2021 AACR Annual Meeting.
3Bratman et al. Nature Cancer. 2020; 1(9):873-881.
Footnotes
*Includes high grade serous ovarian cancer (n=18), endometrial cancer (n=2), and cervical cancer (n=1).