Detect and Monitor ctDNA to Inform Treatment Strategy in HPV-negative and HPV-positive Head and Neck Cancer
Identify
High Risk Patients
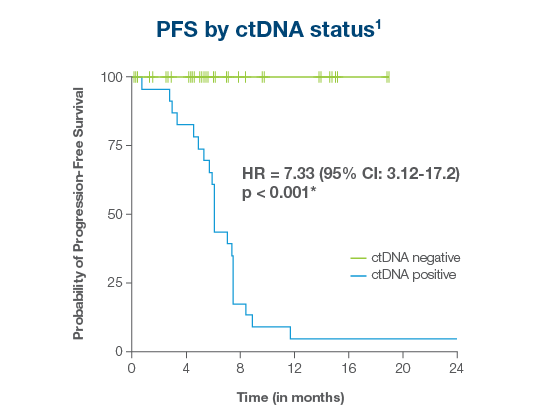
ctDNA-positive patients after definitive therapy achieved just
4.3% PFS
Vs 100% for ctDNA-negative patients1
Detect
Recurrence Earlier
Signatera™ detected recurrence
>4 months
Before standard of care imaging2,3
Monitor Immunotherapy
Treatment Response
ctDNA-positive patients had a
>25x
Higher risk of progression vs ctDNA-negative patients4
Better Biomarkers are Needed to Help Inform Head and Neck Cancer Patient Management



Signatera™ helps inform critical decisions along the continuum of care
Get Started
Identify high risk patients with both HPV-negative and HPV-positive disease
Patients testing Signatera™-positive after therapy experienced a PFS of just 4.3% vs. 100% for Signatera™-negative patients1
- Every HPV-negative patient who tested Signatera™-positive had confirmed persistent or recurrent disease, while every Signatera™-negative patient remained disease free at time of analysis
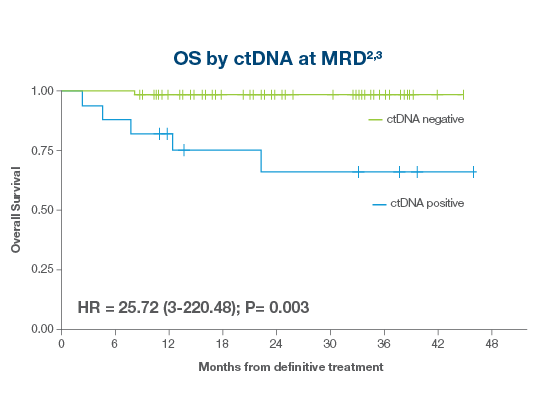
ctDNA-positive patients had a >25x higher risk of death2,3
- HPV-negative and HPV-positive patients testing ctDNA-positive in the MRD window had a >25x higher risk of death, with ctDNA-positive patients experiencing 56% 24-month OS vs 98% for Signatera™-negative patients
Detect recurrence early
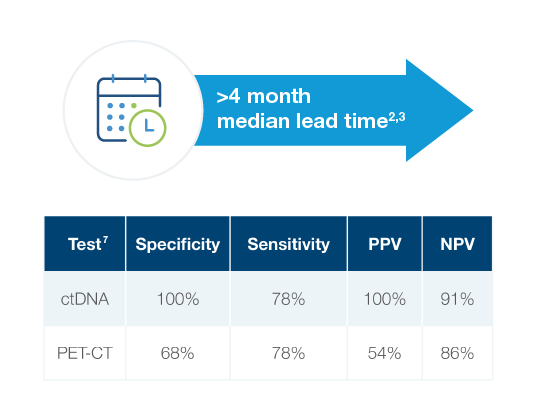
Signatera™ identified head and neck recurrence earlier and more accurately than standard of care imaging2,3
- Signatera™-positivity preceded imaging-confirmed progression by a median of 4.44 months2,3
- Signatera™ outperformed PET-CT in detecting recurrence, showing superior specificity and predictive value7
Predict recurrent/metastatic ICI treatment response
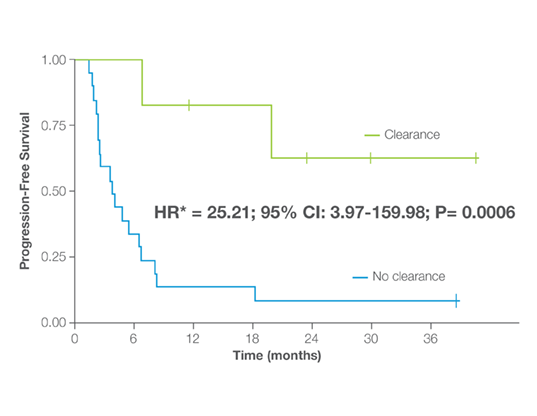
Signatera™ predicted benefit from immune checkpoint inhibitor treatment early, with or without chemotherapy4
- A decrease in Signatera™ ctDNA of ≥20% from baseline to week 6-10 of ICI treatment was associated with clinical benefit
- Every patient who cleared ctDNA achieved a complete or partial response and significantly improved PFS and OS
- ctDNA dynamics were the strongest prognostic factor for PFS in multivariate analysis
Ready to try Signatera™ for your Head and Neck cancer patients?
1Hanna G, et al. Clinical Cancer Research, 2024. DOI: 10.1158/1078-0432.CCR-24-0590.
2Peddada R, et al. Post-treatment head & neck cancer surveillance with ctDNA. Pennington Cancer Institute presentation, 2025.
3Natera. Data on file.
4Honore N, et al. Clinical Cancer Research, 2025. DOI: 10.1158/1078-0432.CCR-25-1309.
5Ionna F, et al. Cancers. 2021 May 14;13(10):2371. DOI: 10.3390/cancers13102371.
6Calandri M, et al. Journal of Thoracic Disease, 2018. DOI: 10.21037/jtd.2018.05.130.
7Lele S, et al. C.-A.O. (2024). Otolaryngology–Head and Neck Surgery, 171:439–444. DOI: 10.1002/ohn.760.