Expanding MRD testing to more patients with colorectal cancer
Giving clinicians Latitude™ when ordering MRD testing
Latitude™ is a tissue-free, blood-based, residual disease test (MRD) that delivers fast and reliable results without the need for tumor tissue. Latitude™ is built using a targeted panel, composed of differentially methylated regions specifically for colorectal cancer (CRC). Latitude™ offers strong performance, with high sensitivity and specificity for detecting residual disease in CRC patients.
Latitude™ Explainer Overview
Latitude™ status post-operatively is highly prognostic
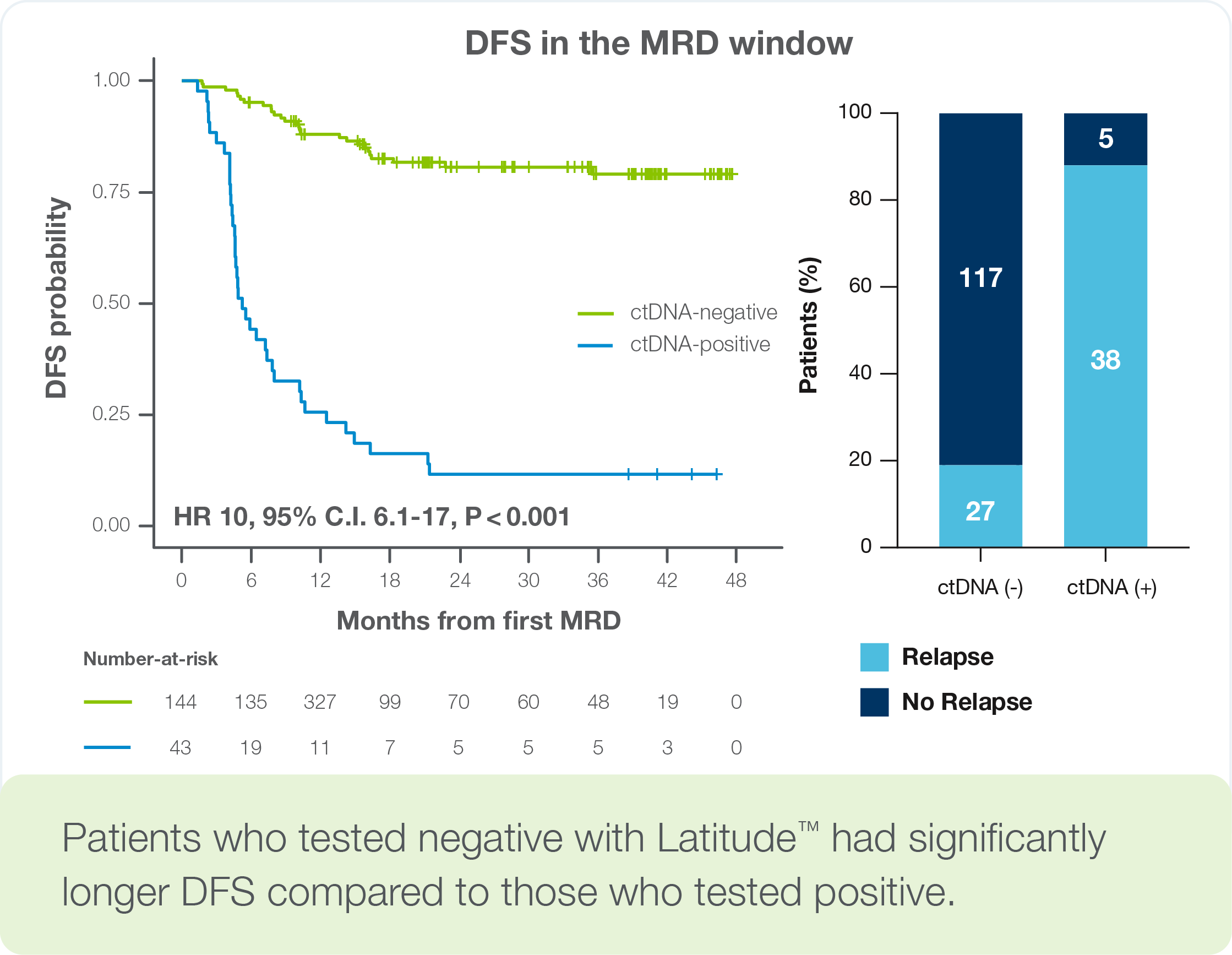
Clinical performance in a 195-patient colorectal cancer study (>1,200 plasma samples) from the CIRCULATE-Japan GALAXY cohort
- Patients who tested negative with Latitude™ had significantly longer disease-free survival (DFS) compared to those who tested positive (MRD window HR = 10.0, p < 0.001)1
- 58% sensitivity at the post-operative MRD timepoint
- 84% longitudinal sensitivity during post-definitive treatment surveillance (87% in colon cancer)
- 97% sample-level specificity
- Median lead time of 4.6 months before radiographic relapse
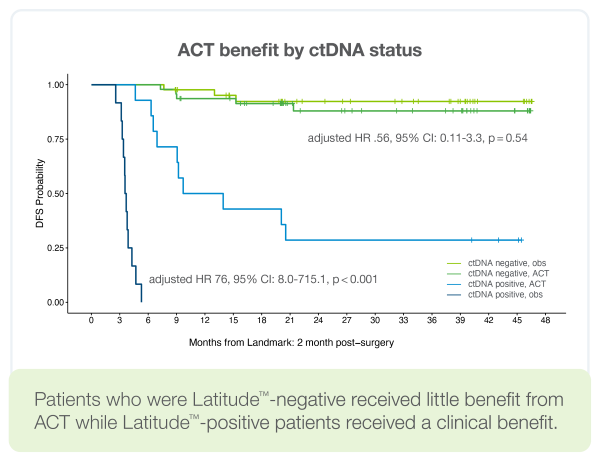
Determine which patients will benefit from ACT
- Patients who were Latitude™-negative derived little benefit from adjuvant chemotherapy (ACT), whereas Latitude™-positive patients experienced a clinical benefit from ACT1
- Latitude™ positive patients who did not receive ACT went on to relapse representing a PPV of 100% (26/26)
How to order Latitude™
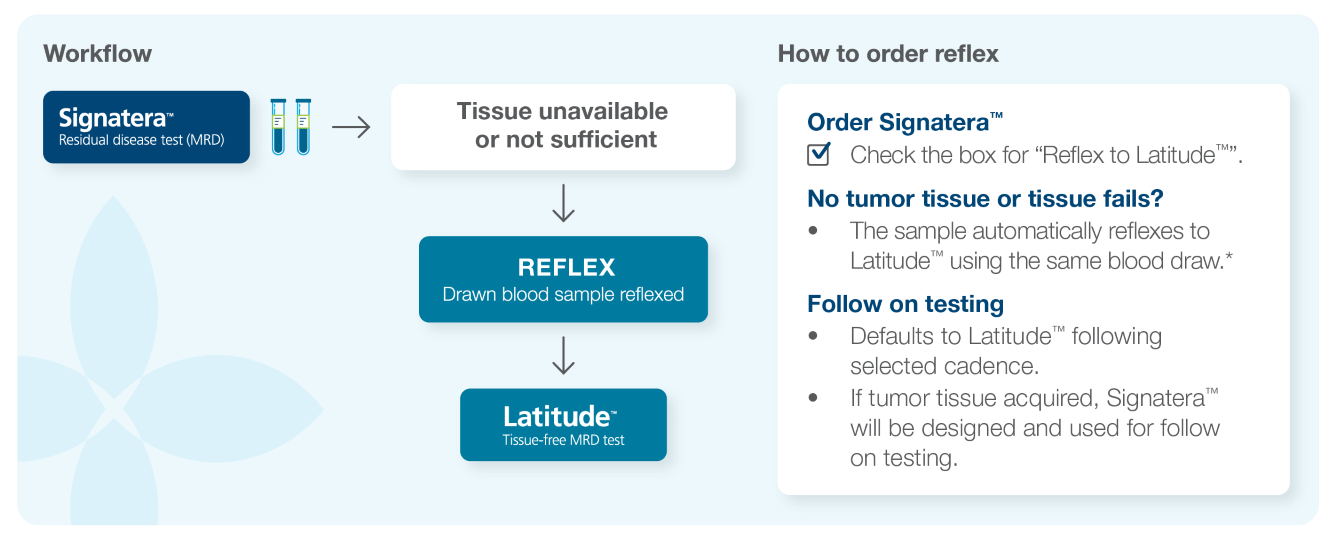
Latitude™ Workflow
Ready to try Latitude™ for your colorectal cancer patients?
1Nakamura Y, et al. Validation of a methylation-based, tissue-free MRD assay in colorectal cancer patients from the GALAXY study. npj Precision Oncology>. 2026.