NateraCore Services Suite
Your Organ Health Support Resource
At Natera, a test is more than just a test; it’s part of an integrated experience, built around a core suite of services to support patients and providers and to make testing easy.
Support every step of the way
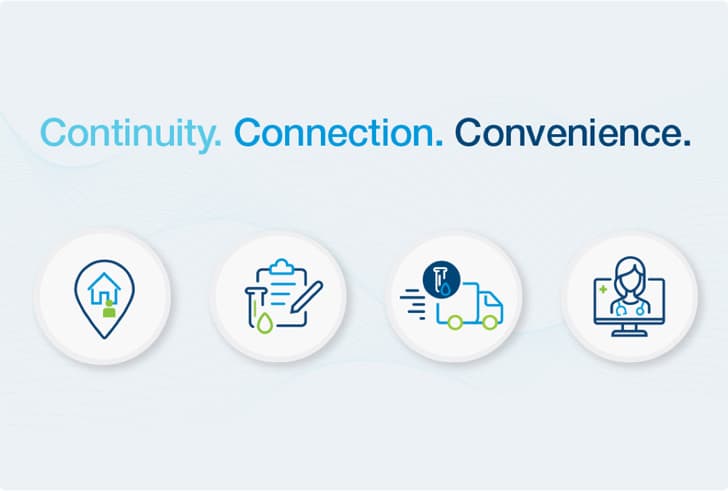
The new ProReach™ Program: Continuity, connection, and convenience in phlebotomy
Leveraging our nationwide mobile blood draw network, this program enables concurrent remote draws of Prospera and other routine labs, delivering phlebotomy truly tailored to your patients.
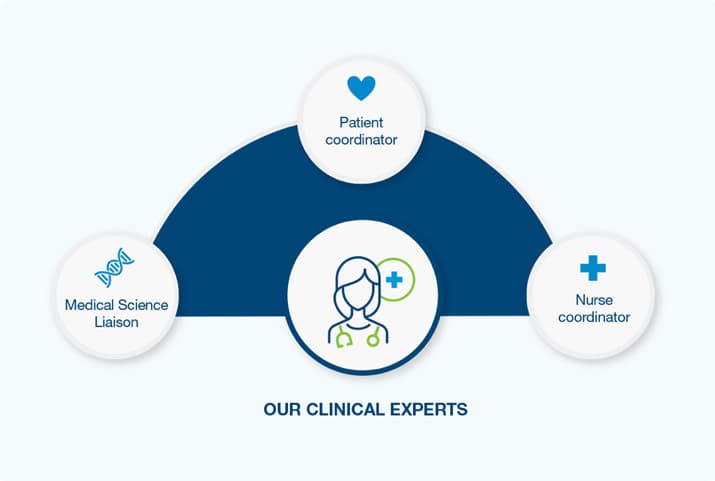
Our Clinical Experts
Natera’s board-certified genetic counselors are available to support your patients with pre- and post-test genetic information sessions.