Renasight™ Case Studies
Discover how Renasight™ delivers meaningful utility and is revolutionizing patient management through genetic insights. Explore real-world case examples demonstrating improved diagnostics, personalized treatment plans, and enhanced patient care.
Case Study: Family History & Patients of African Ancestry – APOL1
Clinical History and Demographics
Patient history
- Recent lab work showed decreased kidney function prompting a nephrology referral for further evaluation
- Urinalysis showed proteinuria that will be monitored
- Diagnosed with CKD stage 2-3
- Patient has a sibling who is on dialysis
- No other family history of renal disease
- Children who have no known kidney concerns
Rationale for genetic test
- Clarify etiology of proteinuria/CKD
- First degree relative with CKD
- Variability in severity within the family
- Assess appropriateness of therapies
- Counsel on likelihood of disease progression
Renasight™ Result – APOL1

APOL1 Clinical Utility:

Identifies etiologic factor of proteinuria/CKD
Can predict accelerated progression of CKD
Consider clinical trial opportunities
Enables patient to benefit from advances in targeted therapies to delay disease progression
Informs risk to relatives
Should transplant be needed, APOL1 testing in living related candidates can be considered
APOL1 status can inform need for future biopsy
Informs recurrence risk should patient require transplantation
Case Study: FSGS – Alport syndrome – COL4A5
Clinical Diagnoses with Genetic Origins
Patient history
- Male patient found to have hematuria at a routine visit with his primary physician as a young adult and referred to nephrology
- Persistent proteinuria developed leading to a renal biopsy that showed FSGS
- CKD stage 4 in his 40s
- Maternal grandfather and a great aunt with history of ESRD
Rationale for genetic test
- Clarify etiology of proteinuria and FSGS
- Maternal family history of disease
- Assess appropriateness of therapies
- Counsel on likelihood of disease progression
Renasight™ Result – COL4A5
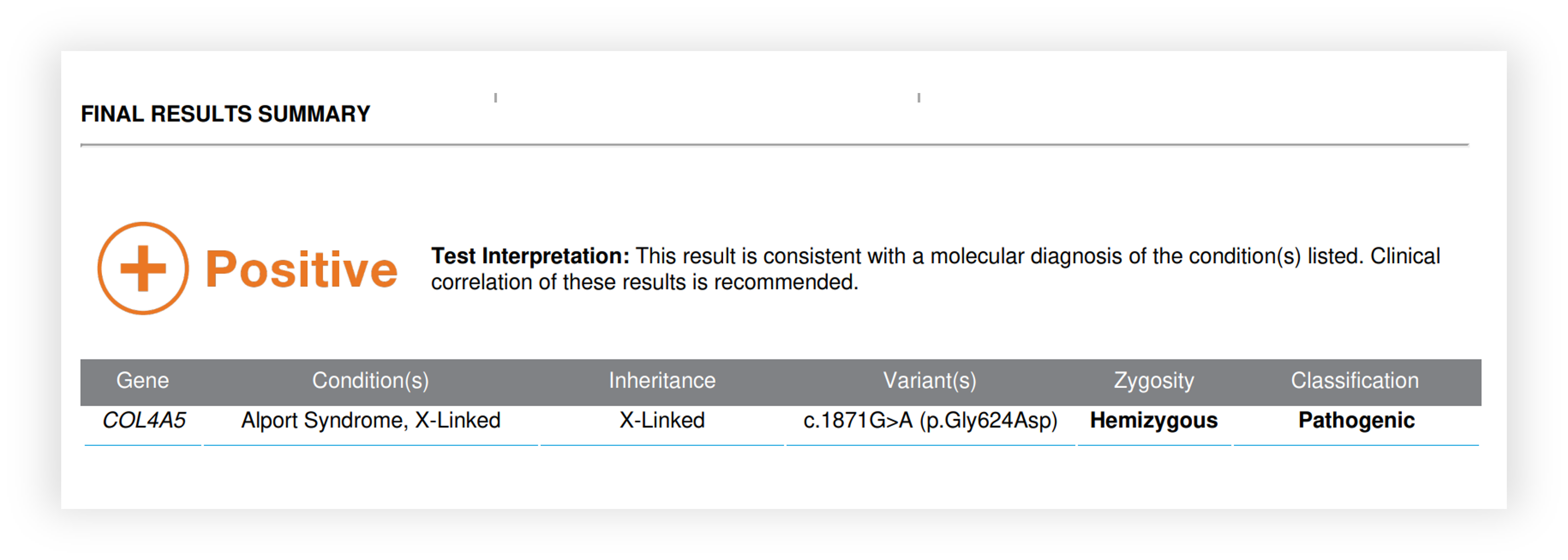
COL4A5 Clinical Utility

Identified cause for proteinuria, CKD and FSGS
Informed risk for progression to ESRD
Identified risk for hearing and vision deficits
Begin treatment with RAAS blockade as guidelines advise
Avoided immunosuppression trials
Identification of at-risk relatives
Informed family planning/ reproductive testing options
Informed on decision to biopsy
Low recurrence risk should patient require transplantation

- Genetic testing is more sensitive and specific than kidney biopsy and is recommended as the gold standard
- When Alport syndrome is suspected, NGS can identify up to 95% of pathogenic COL4A- variants
- Genetic testing is recommended in patients with:
- First degree relative with a COL4A3-COL4A5 variant – this especially includes potential donors
- Persistent hematuria >6 months
- Persistent proteinuria, SRNS, or biopsy-proven FSGS
- Kidney failure without an obvious cause
- Familial IgA glomerulonephritis
Phenotypic spectrum of Alport syndrome
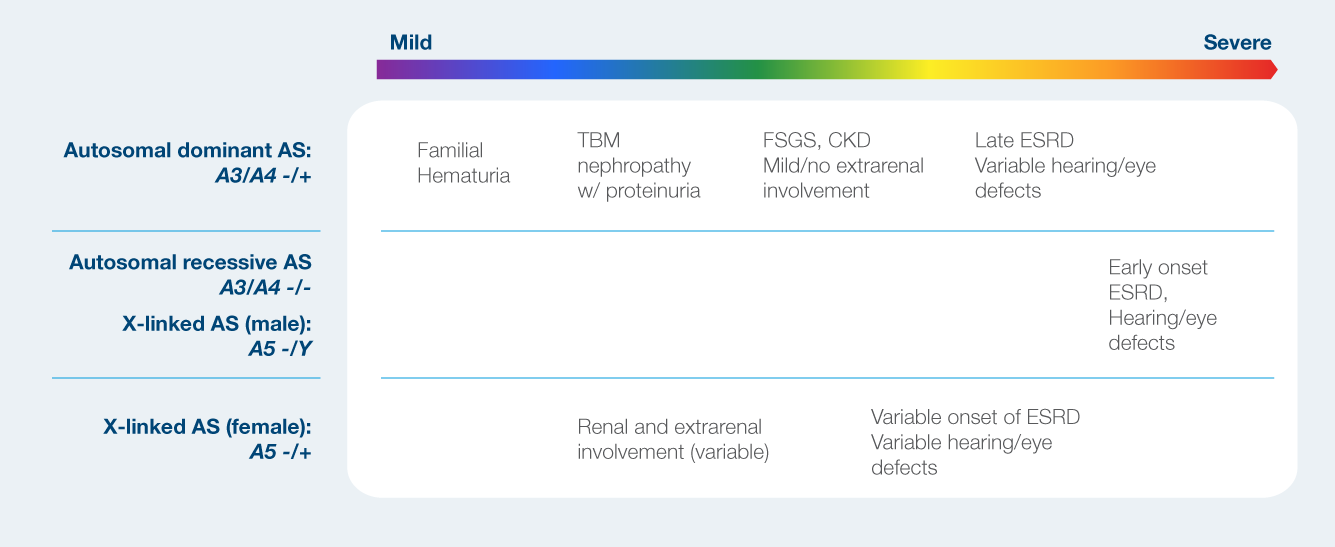
Case Study: Hypertension – UMOD
Unknown/Unclear Cause of CKD
Patient history
- CKD stage 4 attributed to hypertension and NSAID use
- History of gout
- Sibling with renal cysts; no known renal dysfunction
- Father had renal transplant in his 40s
- Another paternal family member had history of ESRD
Rationale for genetic test
- Clarify etiology of CKD
- Several relatives with CKD
- Intrafamilial variability in presentation of CKD
- Assess appropriateness of therapies
- Counsel on likelihood of disease progression
Renasight™ Result – UMOD
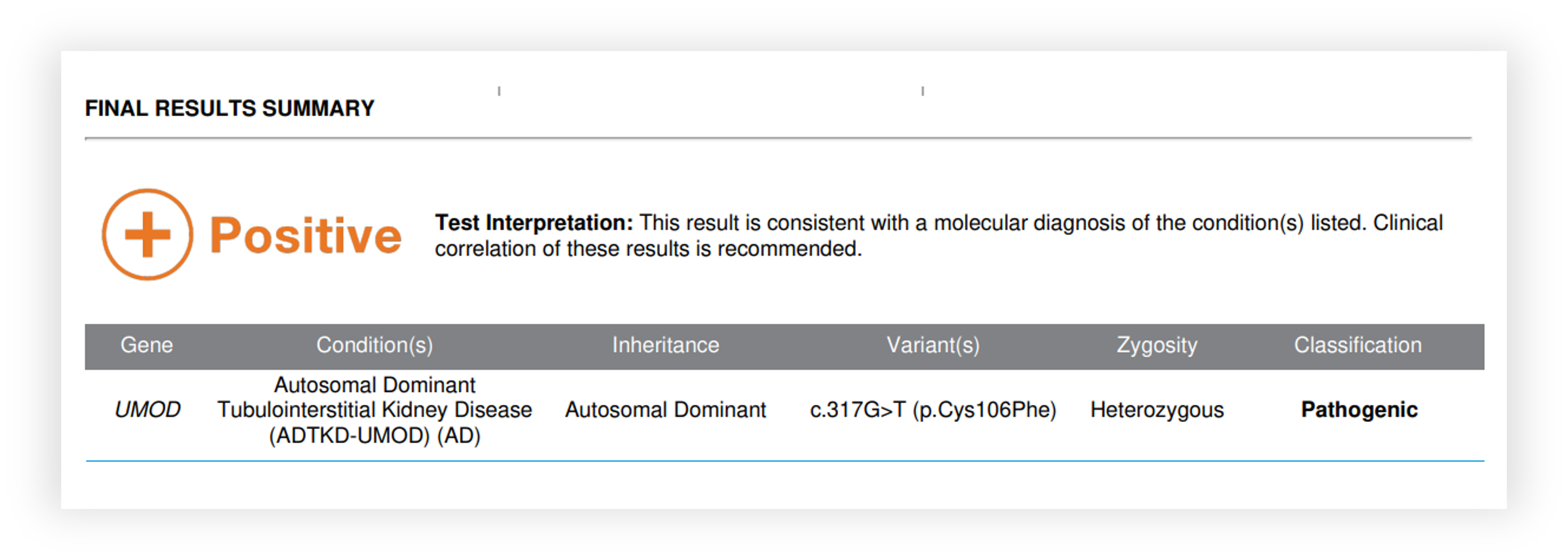
KDIGO Consensus Report on ADTKD
Autosomal Dominant Tubulointerstitial Kidney Disease.
Table 2 | Usual clinical findings in patients with ADTKD
- Autosomal dominant inheritance
- Progressive loss of kidney function
- Bland urinary sediment
- Absent-to-mild albuminuria/proteinuria
- No severe hypertension during early stages
- No drug exposure potentially causing tubulointerstitial nephritis
- Normal or small-sized kidneys on ultrasound
- Nocturia or enuresis in children (owing to loss of renal concentration ability)
Table 3 | Usual findings on renal history in patients with ADTKD
- Interstitial fibrosis
- Tubular atrophy
- Thickening and lamellation of tubular basement membranes
- Possibly tubular dilation (microcysts)
- Negative immunofluorescence for complement and immunoglobulins
"Genetic testing is currently the only way to definitively prove ADTKD and its respective subtypes, and exclude the disease in affected family members"
- UMOD gene accounts for 70% of ADTKD
- Usually inherited (de novo is rare)
- Hyperuricemia, low fractional urate excretion (<5%)
- Early gout for age
- Occasional renal cysts
- Average age of ESRD is 54 yrs (25-70 yrs)
- Intrafamilial variability
- Allopurinol or febuxostat treats gout and hyperuricemia
- No specific renal treatment BUT successful transplant from unaffected donor is considered a cure
UMOD Clinical Utility
Identify risk for progression to ESRD
Inform on decision to biopsy since cause was identified and histologic findings are often nonspecific

Reclassified etiology from HTN/NSAID to one originating from the UMOD gene
Explained the history of gout
Identification of at-risk family members with a 50% recurrence risk
Definitive test for related, eligible donor candidates
Renal transplant from unaffected donor is considered a cure
Case Study: Transplant Candidate Informing Donor Eligibility – HNF1B
Transplant candidate with cystic disease
Patient history
- CKD stage 5, renal cysts, hypertension
- Diagnosed with ADPKD
- Family history unknown
Rationale for genetic test
- Clarify etiology of CKD
- Assess appropriateness of therapies
- Anticipate pre and post transplant course
Renasight™ Result – HNF1B
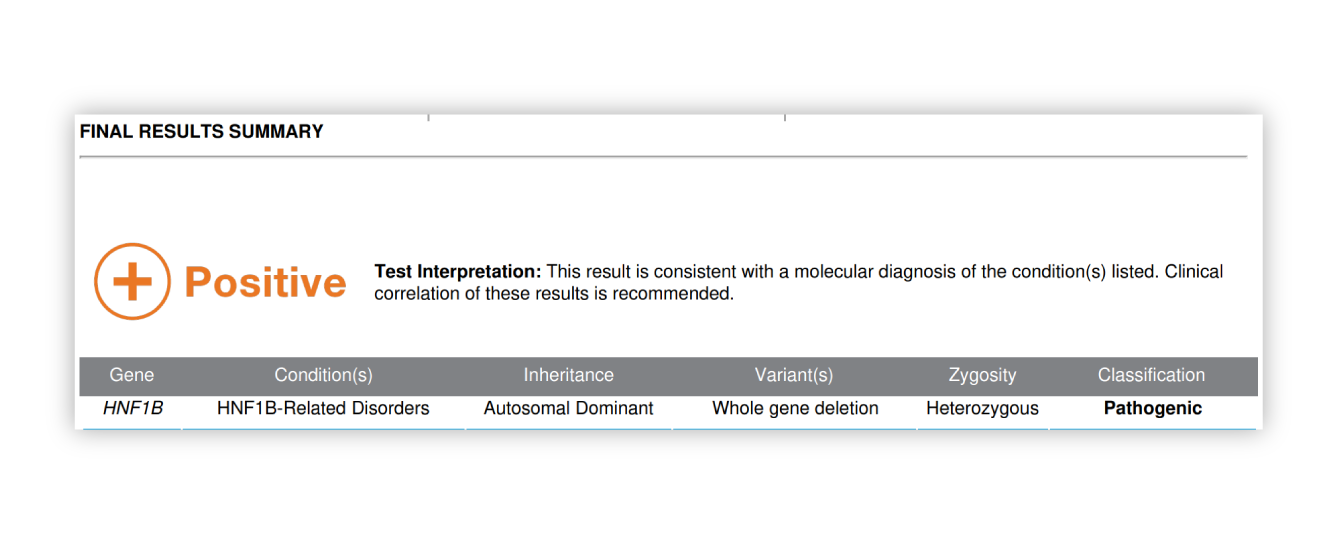
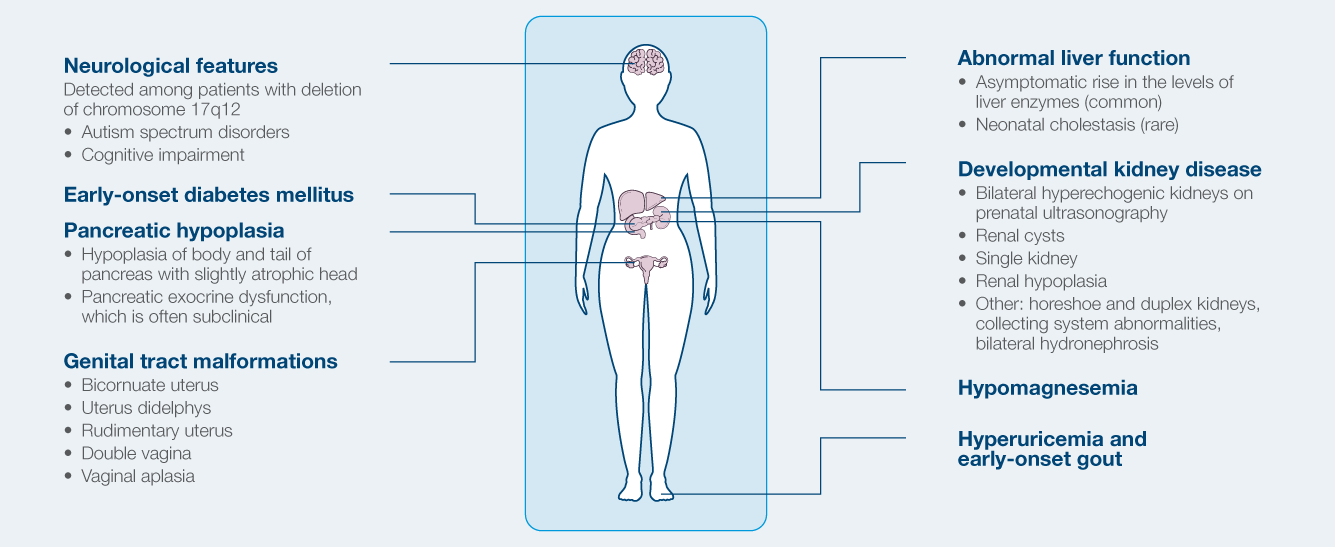
Phenotypic spectrum of HNF1B-related ADTKD
- Formerly referred to as Renal Cysts and Diabetes syndrome
- Most common known monogenic cause of developmental renal disease
- Strong intrafamilial variability
- Can manifest in prenatal period (agenesis/hypoplasia)
- Cystic disease in 73%
- Diabetes develops in ~50%
- Electrolyte abnormalities
- Hypomagnesemia, hyperuricemia (gout)
- 50% risk to children
HNF1B Clinical Utility
Identify risk for multiple extra-renal features

Inform on treatment given nonclassic form of PKD
Identification of at-risk family members given 50% risk of recurrence
Test available for eligible donor candidates in which intrafamilial variability is common
Increased risk of new-onset diabetes after transplant (NODAT), IS regimen that avoids tacrolimus and reduces corticosteroid exposure
Case Study: Patient with clinical diagnosis of ADPKD – PKD1
Patient history
- Male in early 30s
- Kidney cysts identified incidentally in late 20s during imaging for a back strain
- HTN, normal kidney function
- No family history of PKD
- PROPKD score: 3 (low)
Rationale for genetic test
- Due to patient’s young age and no family history, provider is unsure about severity of disease and risk of progression
- Should this patient be offered treatment with Tolvaptan?
Renasight™ Result – positive for PKD1 truncating variant

PKD1 Clinical Utility
Patient's PROPKD score with PKD1 truncating variant is now 7 - HIGH RISK
Patient is at risk for extrarenal features of PKD - screening started for aneurysm and cardiac issues

Tolvaptan treatment started (can have 35% reduction in decline of kidney function)
Allows for accurate assessment of reproductive risk (50% risk of ADPKD per pregnancy) and discussion of options (e.g., IVF/PGD)
Patient is considering genetic testing for future pregnancies with partner (50% risk of ADPKD)
Disease not expected to recur in graft
Family members considering kidney donation can be tested for familial variant --especially useful for affected siblings who may still have normal imaging.
Case Study: Patient with a few kidney cysts ADPKD or sporadic? – IFT140
Patient history
- Female in early 40s
- A few kidney cysts identified incidentally via ultrasound
- 2 cysts in L kidney, 1 large cyst in R kidney
- Kidney volume and function normal
Rationale for genetic test
- Unsure whether patient’s ultrasound findings represent sporadic cysts or polycystic kidney disease
Renasight™ Result – positive for IFT140
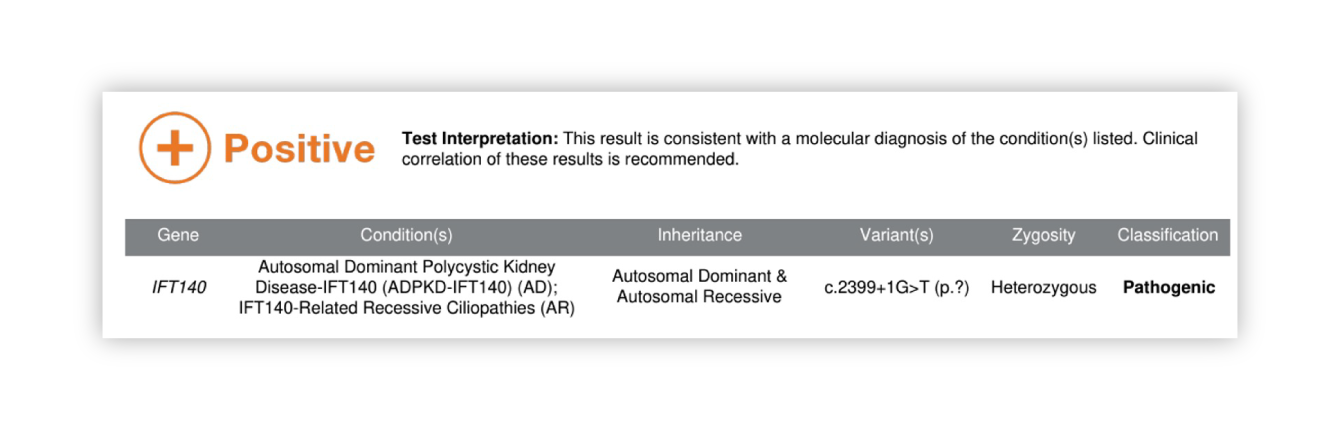
IFT140 Clinical Utility
Patient has a new diagnosis of atypical ADPKD
ADPKD-IFT140 is typically more mild, lower risk of progressive CKD
Risk of extrarenal features is lower, still warrants surveillance

Patient should be screened over time
Tolvaptan deemed not to be indicated, given result in context of normal kidney volume & function
As ADPKD-IFT140 is typically milder, family member may not be aware they are affected
Patient’s parents have genetic testing, her mother is found to be affected and starts surveillance
If patient needs transplant, family members can be screened with genetic testing. Imaging is not ideal as affected individuals may have no or very few cysts
Transformative impact: outcomes from Renasight™
Case Study: Patient with long-standing hematuria – COL4A4
Patient history
- Male in mid-40s
- Longstanding microscopic hematuria, first identified in sports physical as a teen
- Recent eGFR is 62 – CKD stage 2
- Family history of hematuria in patient’s mother
Rationale for genetic test
- Provider considering kidney biopsy to help determine etiology and prognosis
- Patient has reservations about undergoing biopsy
Renasight™ Result – positive for AD Alport syndrome
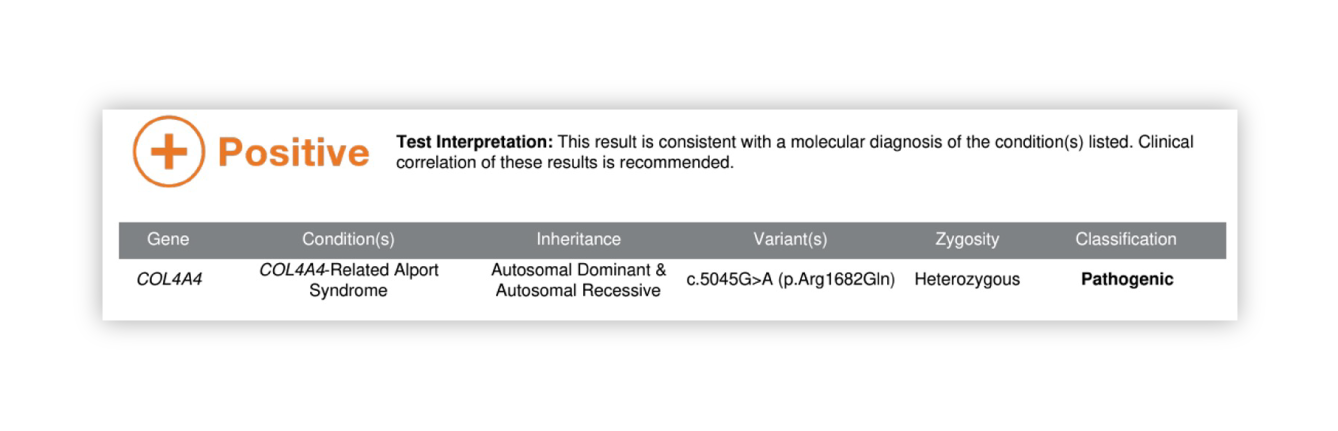
COL4A4 Clinical Utility
Result explains patient’s hematuria and CKD
Appropriate screening for extra-renal features
Biopsy avoided
Extra renal features

RAAS blockade treatment
Avoid treatment with steroids
Clinical trials for Alport treatment
Patient’s mother who had hematuria is tested and is positive and found to have CKD3
Patient’s siblings and children at 50% risk; family members can consider testing
If patient needs a transplant, family members (at 50% risk, but may not show symptoms yet) can be screened
Post-transplant recurrence is unlikely
Case Study: Benign familial hematuria – COL4A5
Patient history
- Female in early 50s with long history of hematuria
- Normal eGFR
- Biopsy done in her 30s after proteinuria in pregnancy
- Told she has ‘benign familial hematuria’ and not to worry
- Her teenage son, recently found to have hematuria on sports physical and workup, shows decreased eGFR
Rationale for genetic test
- Provider has suspicion about genetic etiology given the son’s early age of onset
Renasight™ Result – positive for X-linked Alport syndrome

COL4A5 Clinical Utility
New diagnosis of X-linked Alport for patient - women typically less severely affected but are at risk for progressive CKD
Risk of hearing loss and vision issues
Cardiac risk of aortic dilation

RAAS blockade treatment
Avoid treatment with steroids
Clinical trials for Alport syndrome
Son is expected to be affected - likely to experience ESRD
Daughters at 50% risk, need testing to determine their risk and risk to their children
Affected daughters may consider pregnancy planning options
Son will likely need a kidney transplant, family members can have genetic screening for donor eligibility
Recurrence risk in graft is low
Avoid immunosuppression with steroids
Case Study: Patient with ADPKD needing kidney transplant – PKD1
Patient history
- Female in mid-50s
- Longstanding clinical diagnosis of PKD
- Family history of affected father and uncle, both deceased
- Patient is on dialysis and hoping to receive a kidney from one of her children
Rationale for genetic test
- In order to screen the patient’s young children who at 50% risk, but may not have formed cysts yet, provider needs to find the cause of PKD in the patient
Renasight™ Result – positive for PKD1 truncating variant
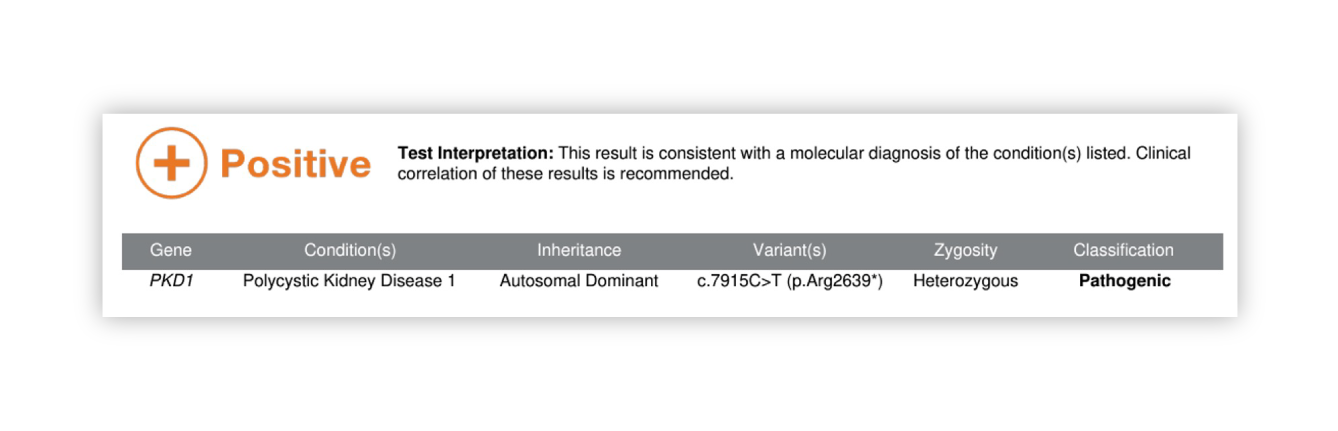
PKD1 truncating variant Clinical Utility
Confirms patient’s clinical PKD diagnosis
PKD1 truncating variant is consistent with patient’s severe disease

Clinical treatment not changed as patient has ESRD
Treatment is a kidney transplant, family members can now be tested for donation
Patient’s children are at 50% risk and due to their young ages (18, 20, 22) imaging is not sufficient to rule out ADPKD
Children are tested
1 child is positive and initiates management and Tolvaptan
2 children are negative for familial variant and can be worked up for possible donation
Case Study: Patient with ADPKD needing kidney transplant – PKD2 & COL4A4
Patient history
- Male in early-50s
- Recently clinically diagnosed with ADPKD via imaging
- Patient presented to ED with ESKD, imaging identified cystic, enlarged kidneys
- Family members want to be screened for possible donation, proceed with kidney ultrasound
Rationale for genetic test
- Patient has one child who is found the have multiple kidney cysts and cannot donate
- Patient has two children who do not have cysts. Both are being worked up for donation
- Provider decides to do genetic testing as patient is highly concerned about one of his children donating a kidney
Renasight™ Result – dual-positive for PKD2 and AD Alport syndrome
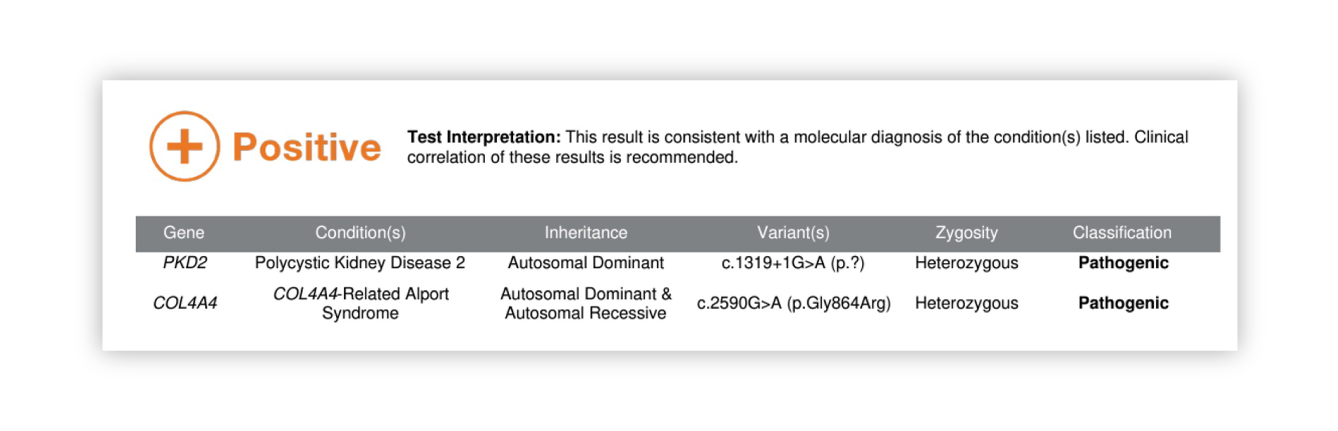
Dual-positive for PKD2 and AD Alport syndrome Clinical Utility
Patient has ADPKD AND AD Alport
Patients with AD Alport typically have hematuria and are at risk for CKD, as the AD form can be more mild, it is common for this diagnosis to be missed
AD Alport may explain patient’s progression which is more severe than expected for PKD2
Risk of hearing loss and vision issues

Clinical management not changed as patient has ESKD
Treatment is a kidney transplant, family members can now be tested for BOTH conditions for donation eligibility
Patient’s children are at 50% risk for ADPKD and also 50% risk for AD Alport
All family members (those with and without PKD) need screening for AD Alport
Family members who have PKD and or Alport can be proactively treated to help protect kidney function
Neither disease is expected to recur in the graft
Both of the patient’s children who didn’t have kidney cysts are negative for PKD2, one has Alport and can now evaluate the risks of donating a kidney
Case Study: Patient with clinical ADPKD – UMOD
Patient history
- Male mid 40s
- Patient reports a clinical diagnosis of ADPKD, he has bilateral kidney cysts and reported a family history of PKD
- Has a family history of multiple people with ‘CKD and kidney cysts’
- Patient has a consistently declining eGFR, but his kidney cysts are relatively stable
Rationale for genetic test
- The patient’s decline in kidney function is typical for ADPKD, but his imaging doesn’t look typical for his level of progression. Is this an atypical PKD?
Renasight™ Result – positive for ADTKD-UMOD
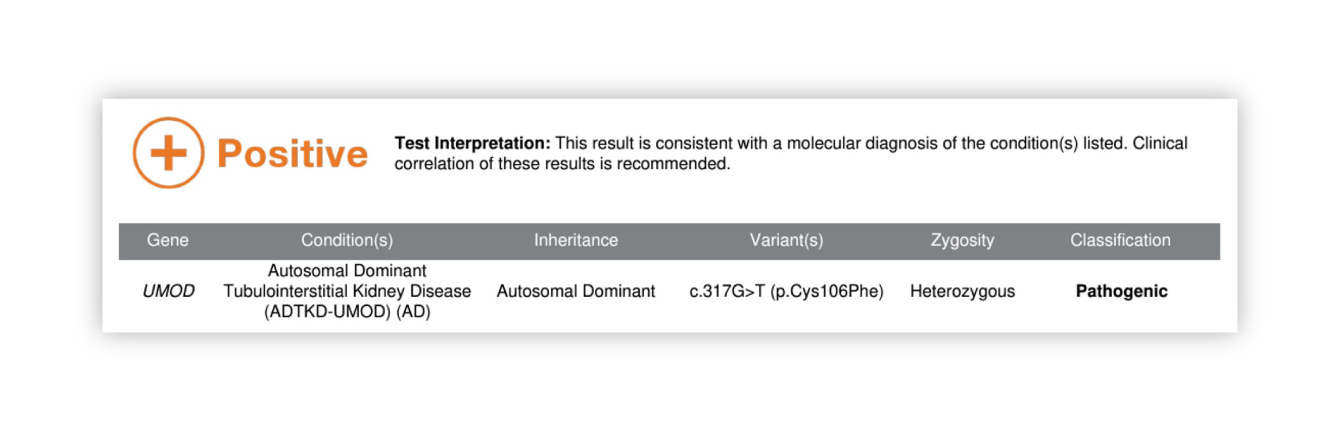
ADTKD-UMOD Clinical Utility
Patient’s diagnosis is reclassified to ADTKD-UMOD
Different extrarenal risks, discontinue aneurysm screening, initiate gout screening
Prognosis is more variable with UMOD

Change in treatment options. Potential treatment with allopurinol
Low salt diet NOT recommended, consider low purine diet
UMOD is more clinically variable than PKD. Family members who had been screened via imaging and were thought to be unaffected may be affected with UMOD and at risk for progressive CKD
Family members can have genetic testing and appropriate diagnosis and management
Screening potential donors via imaging would not have been sufficient, now family members can be screened via genetic testing
Disease is not expected to recur in the graft, but there is less data for ADTKD
Case Study: CKD of unknown etiology – SALL1
Patient history
- Male early 20s
- CKD stage 2, proteinuria, and mild hearing loss
- No known family history of kidney issues
Rationale for genetic test
- Clarify unknown etiology of kidney disease in a young patient
- Provider suspects Alport syndrome due to hearing loss
Renasight™ Result – positive for SALL1
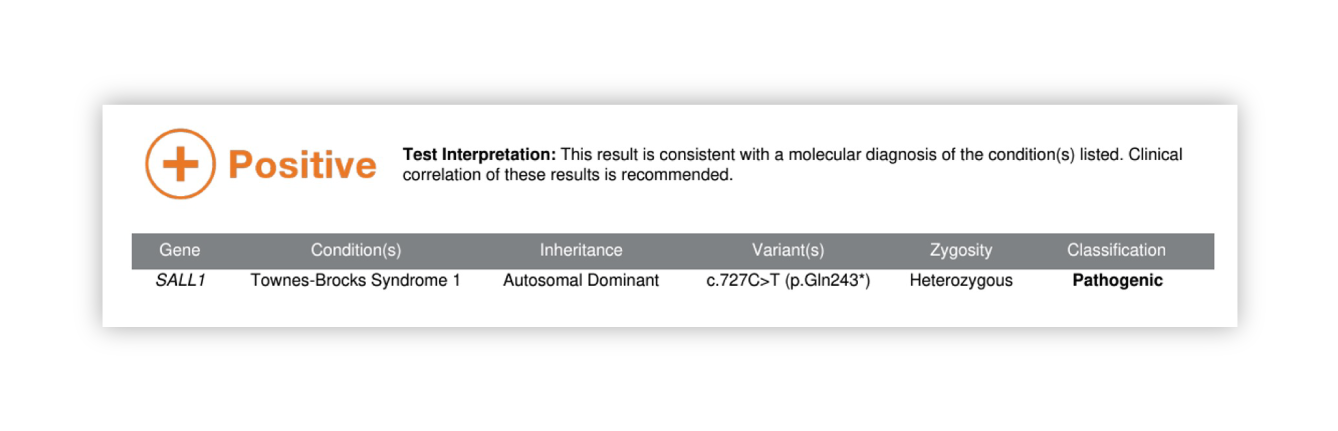
SALL1 Clinical Utility
New diagnosis of a condition that was not on the provider’s differential
As patient doesn’t have the classic triad of features, directed testing may never have been done
Imaging done for CAKUT

CKD management
Refer to genetics for workup for extrarenal features
50% of cases are new, 50% are inherited. Family members can have screening
Patient is interested in embryo testing for pregnancy planning to avoid passing on to a child as it could be more severe
Disease should not reoccur in a transplanted kidney
Family members can be tested if patient needs transplant
Case Study: CKD in a patient of African ancestry – APOL1 & COL4A3
Patient history
- Male late 20s
- Patient presented with CKD stage 3
- History of HTN
Rationale for genetic test
- Provider is suspicious about APOL1 given patient’s ancestry and disease progression at a younger age
Renasight™ Result – positive for APOL1 & AD Alport syndrome
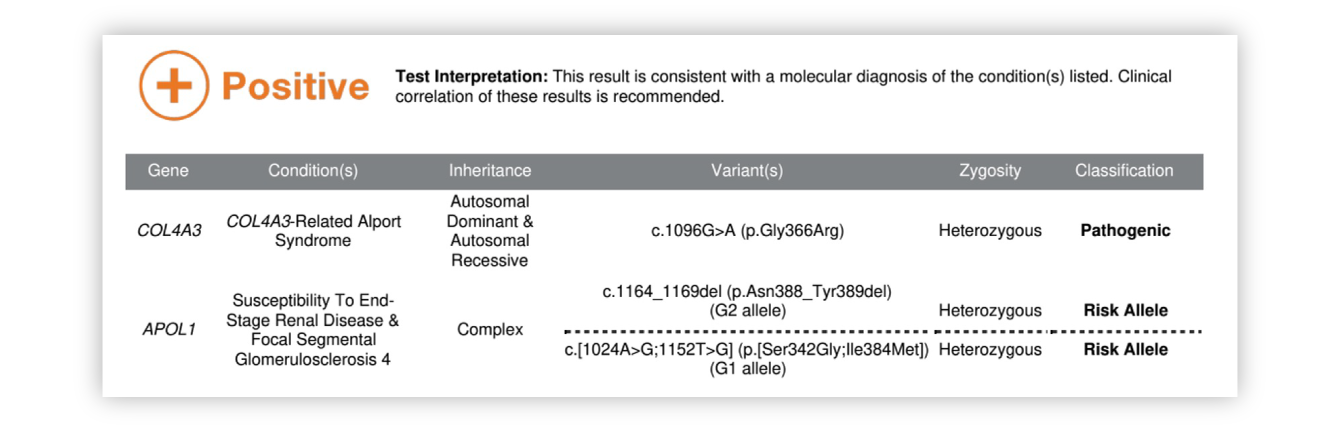
APOL1 & AD Alport syndrome Clinical Utility
Dual diagnosis of APOL1 and AD Alport could explain CKD at young age
Patient is at risk of ESRD
Extrarenal features include SNHL and vision issues

ACE inhibitor
Avoid treatment with steroids
Consideration of clinical trials for AAPOL1
Family members are at risk for both APOL1
Genetic counseling can define risks to family members and help with testing
Transplant is reasonable. APOL1 can recur in the graft, Alport should not recur.
Screening for possible familial donors for both conditions
Case Study: ‘Hypertensive nephritis’ – CYP11B1 & CYP11B2
Patient history
- Female early 20s
- CKD2
- HTN first identified at age mid teens
- HTN has been difficult to control, multiple medications have been tried
- Family history of father and uncle both with long standing HTN. Father has CKD4, uncle has ESR
Rationale for genetic test
- Provider thinks patient’s CKD is due to HTN, but wonders if there may be something genetic going on
Renasight™ Result – positive for CYP11B1/CYP11B2 fusion
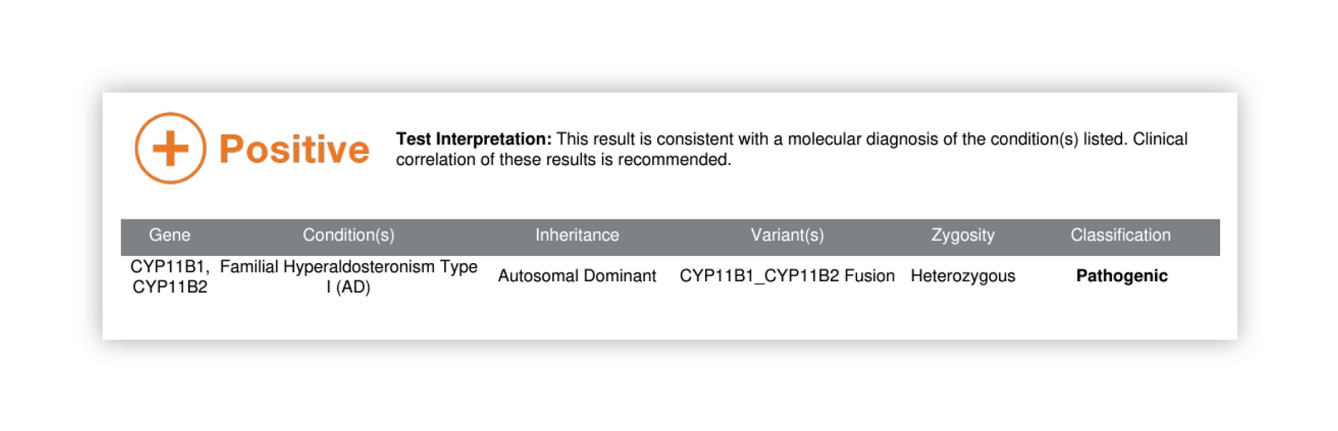
CYP11B1/CYP11B2 fusion Clinical Utility
The patient’s HTN has a rare, monogenic cause and is treatable. With early treatment, progression of kidney disease can be slowed or stopped

Instead of typical HTN medication, recommended treatment is glucocorticoids.
The patient’s father and uncle can have testing done and their medication can be changed if they have the same diagnosis
The patient has not had children yet. She plans to pursue embryo testing now that the cause has been identified
Affected family members needing a transplant should be treated or disease could recur in a transplanted kidney
Allows for testing of potential related donors
Case Study: Proteinuria of unknown etiology with extrarenal features – GLA
Patient history
- Female mid 40s
- Presents to nephrology for significant proteinuria
- Creatinine is WNL and eGFR remains >60
- Diagnosed with diabetes following the birth of her son 10 years ago, but this has been reasonably well-controlled
- Patient notes a history of GI issues with chronic constipation
- Also reports periodic numbness in her arms and occasional leg pain, as well as tinnitus
Rationale for genetic test
- Clarify the etiology of patient’s proteinuria before proceeding to biopsy
- Provider is curious whether the patient’s extrarenal features may be related in some way
Renasight™ Result – positive for Fabry disease (GLA)
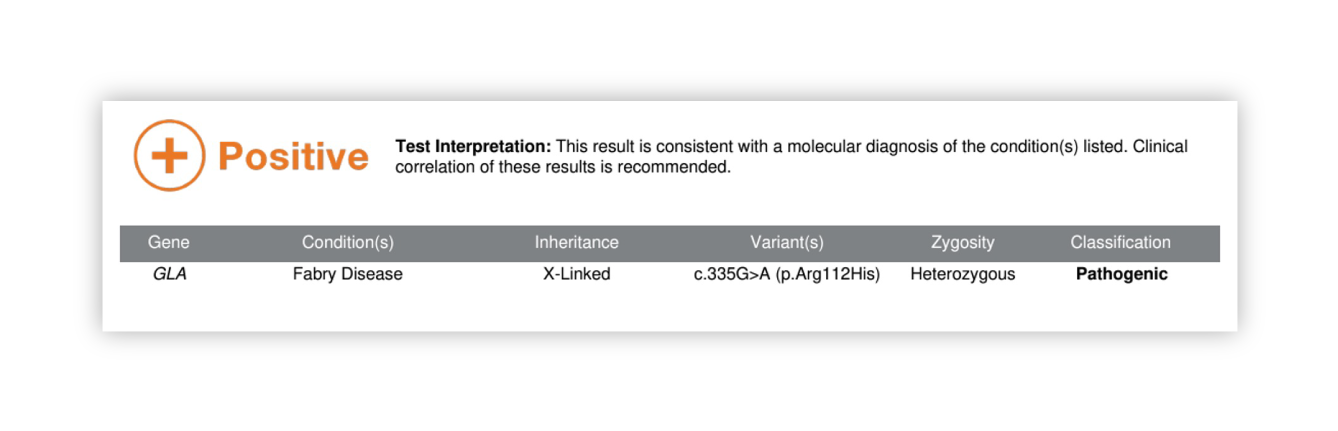
Fabry disease (GLA) Clinical Utility
No need for biopsy
Significant risk for extrarenal features including cardiovascular disease

Targeted therapy is available via ERT with or without chaperone therapy
Identification of at-risk relatives, including the patient’s 10 yo son with unexplained GI issues and foot pain
ERT recommended as early as possible in all males and in females with significant symptoms
Allows for testing of potential related donors, particularly asymptomatic females
Case Study: Patient with clinical diagnosis of ADPKD – HNF1B
Patient history
- Male mid 40s
- Patient reports a clinical diagnosis of ADPKD, he has bilateral kidney cysts and reported a family history of PKD
- Has a family history of multiple people with ‘CKD and kidney cysts’
- Patient has a declining eGFR, but kidney cysts are relatively stable
- Patient was recently diagnosed with type II diabetes
Rationale for genetic test
- Understand the reason for declining eGFR in a patient with stable kidney cysts
- Understand the family history of CKD and cysts
Renasight™ Result – positive for HNF1B
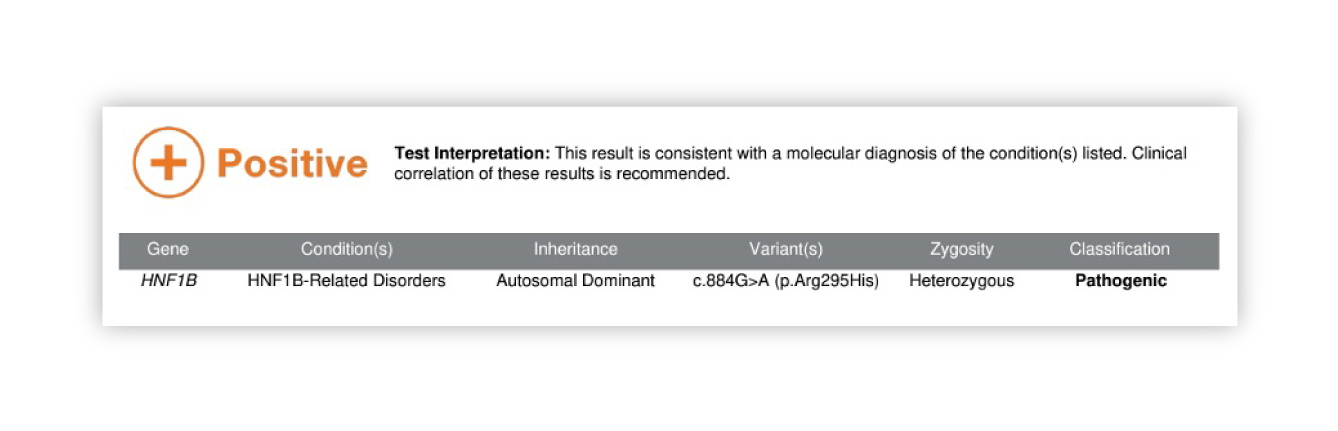
HNF1B Clinical Utility
Patient’s diagnosis is reclassified to ADTKD-HNF1B
Different extrarenal risks, discontinue aneurysm screening, initiate gout screening
Prognosis is more variable with HNF1B compared to PKD1

Change in treatment options
Type II diabetes is reclassified to MODY and medication is changed to sulfonylurea
HNF1B is more clinically variable than PKD. Family members who had been screened via imaging and were thought to be unaffected may be affected with HNF1B and at risk for progressive CKD
Family members can have genetic testing and appropriate diagnosis and management
Clarification of HNF1B diagnosis is very important for transplant
Avoidance of steroids and calcineurin inhibitors post transplant which would increase risk of developing diabetes post transplant
Disease not expected to recur in graft
Screen family members for possible kidney donation