Now available for 21 genes
Fetal Focus™, the next-generation single-gene NIPT
Powered by ultra-sensitive LinkedSNP™ technology.
Backed by robust validation in the prospective EXPAND clinical trial.

LIVE WEBINAR
Fetal Focus™:
The next-generation single-gene NIPT enables actionable interventions
Session 1: Tuesday, February 17th @ 10 AM PT
Session 2: Thursday, February 19th @ 2 PM PT
Upcoming Webinar
Fetal Focus™, the next-generation single-gene NIPT, is now available for up to 21 recessive and X-linked conditions. By directly screening the fetus using cfDNA from the pregnant Horizon™ carrier’s sample, Fetal Focus™ provides fetal risk insights without requiring a partner’s sample.
Hear from Dr. Lee Shulman and Dr. Jeffrey Meltzer as they discuss the clinical utility of Fetal Focus™ in enabling earlier diagnosis and treatment of inherited single-gene conditions.
Direct fetal risk assessment for 21 recessive and X-linked genes
By directly screening the fetus for inherited single-gene conditions using cfDNA, Fetal Focus™ addresses challenges with standard carrier screening when the partner does not complete testing. The test screens for ACOG-recommended conditions like cystic fibrosis and sickle cell disease,1 and now includes up to 21 autosomal recessive and X-linked genes.
Early Interventions for Conditions Screened by Fetal Focus™
FDA-approved
interventional treatments
- Cystic fibrosis (CFTR)
- Spinal muscular atrophy (SMN1)
- Alpha-thalassemia (HBA1/2)
- Beta-hemoglobinopathies including sickle cell disease (HBB)
- Duchenne muscular dystrophy (DMD)*
- Glycogen storage disease Type 2 (Pompe disease) (GAA)
- Krabbe disease (GALC)
- Gaucher disease (GBA)
Dietary modifications
or supplements
- Medium-chain acyl-CoA dehydrogenase deficiency (ACADM)
- Phenylketonuria (PAH)
- Smith-Lemli-Opitz syndrome (DHCR7)
- Carnitine palmitoyltransferase II deficiency (CPT2)
- Galactosemia (GALT)
- Familial mediterranean fever (MEFV)
Management that reduces
complications or supports development
- Canavan disease (ASPA)
- Tay-Sachs disease (HEXA)
- Familial dysautonomia (IKBKAP)
- Fragile X (FMR1)*
- Polycystic kidney disease, autosomal recessive (PKHD1)
- Wilson disease (ATP7B)
Bolded text indicates Fetal Focus™ is the only single-gene NIPT that offers fetal cfDNA screening for this gene.
Fetal Focus™ is available for patients screened with Horizon™ 4, Hbasic, H14, H27, H106, and H274.
*Fetal risk assessment for X-linked conditions is based on fetal sex only. Fetal Focus™ does not directly screen the fetal DMD or FMR1 genes
Proven performance in the landmark EXPAND study

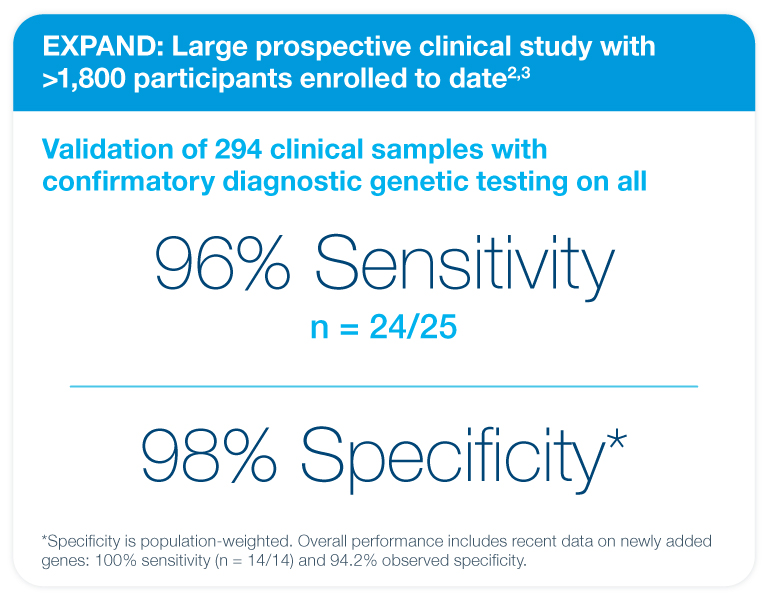
Fetal Focus™ is a screening test, not a diagnostic test. False positive and false negative results can occur. A high risk result does not mean the fetus is affected. Similarly, a low risk result does not definitively mean the fetus is unaffected. Genetic counseling and diagnostic testing should be offered to further evaluate high risk results.

Having access to a noninvasive option like Fetal Focus™ can provide critical information to support decision-making during pregnancy, especially in situations where partner testing isn’t possible
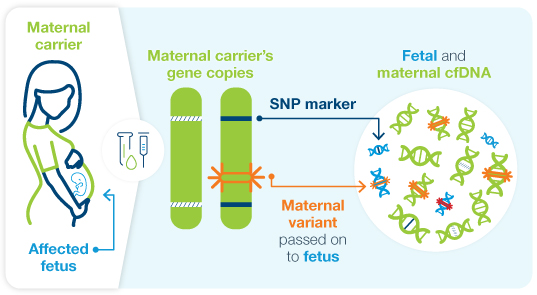
Powered by ultra-sensitive LinkedSNP™ technology
Fetal Focus™ combines direct variant detection with our ultra-sensitive LinkedSNP™ technology to assess if the fetal variant was inherited from the mother or father. This innovation enables improved detection of affected pregnancies across diverse populations.
Designed to integrate into your workflow with ease



*Fetal Focus™ is available with Horizon a la carte (individual orders of cystic fibrosis and spinal muscular atrophy), H4, Hbasic, H14, H27, H106, and H274
Available with the #1 ordered NIPT and carrier screen4
In one blood draw, your patients receive the pioneering SNP-based Panorama™ NIPT and comprehensive Horizon™ carrier screening with Fetal Focus™ as an add-on

Learn more about the next‑generation Fetal Focus™ sgNIPT
1American College of Obstetricians and Gynecologists, Committee Opinion #691, March 2017
2Internal EXPAND validation data. In EXPAND, the study participants and investigators are blinded to the Fetal Focus™ test results. 12/294 samples did not receive a result.
3Expanding Prenatal Cell Free DNA Screening Across MoNogenic Disorders (EXPAND). https://clinicaltrials.gov/study/NCT06808880.Accessed December 2025.
4Internal analysis of Denitive Healthcare database and ancillary data. Dec 2023.