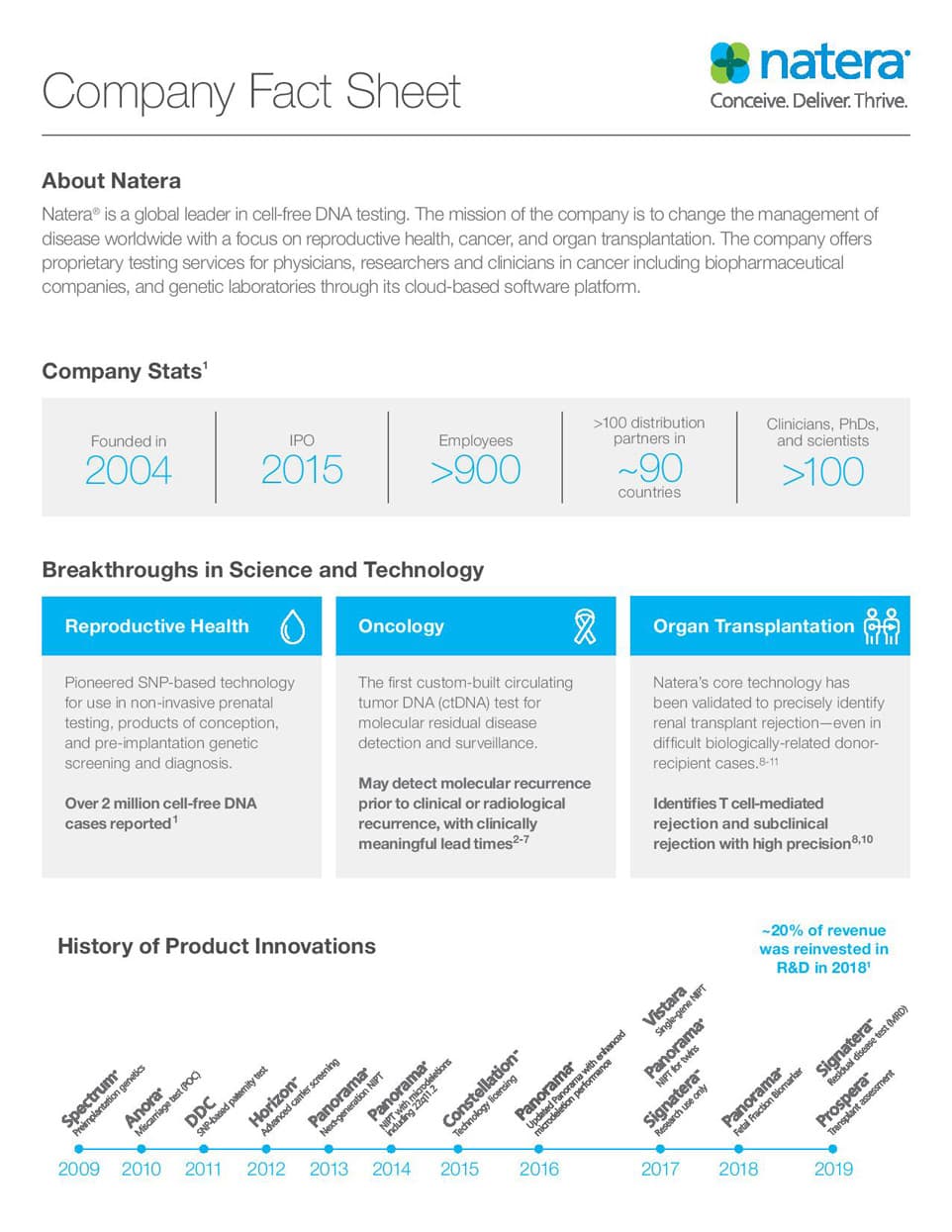
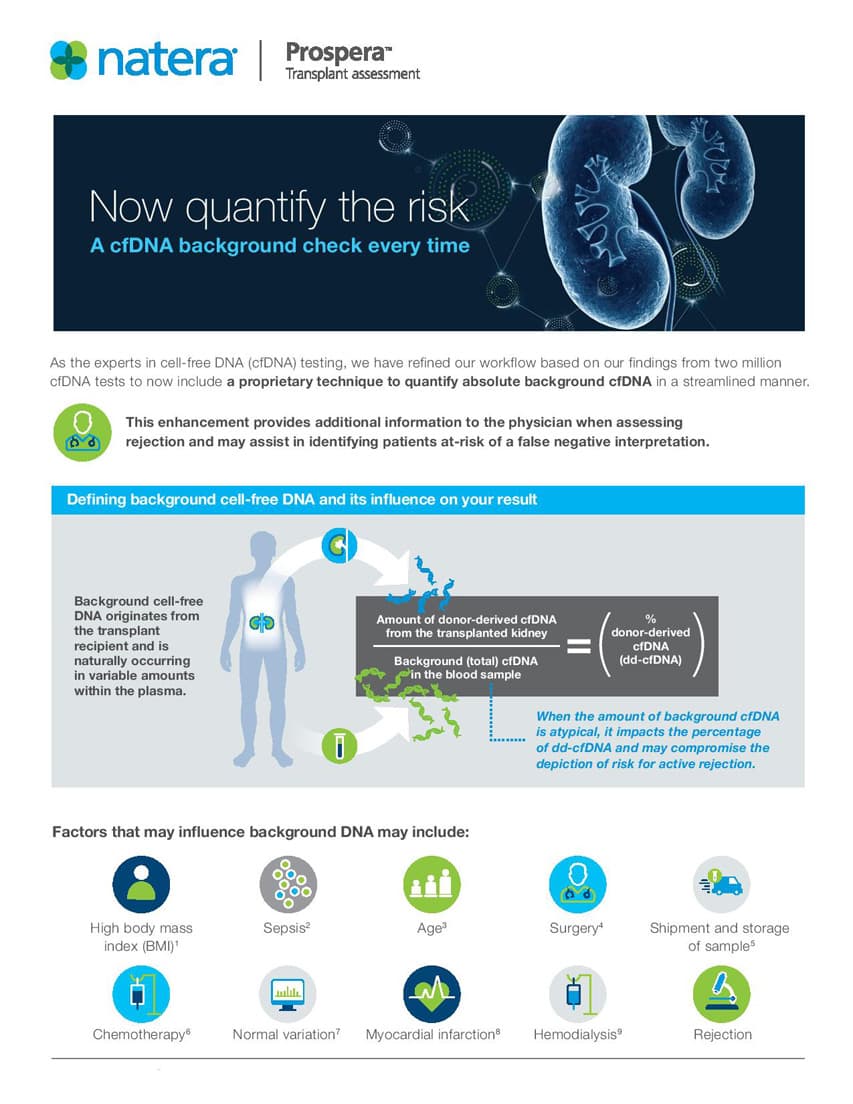
Now quantify the risk
Run a cfDNA background check every time
Prospera™ Precision
- Prospera Transplant Assessment with Quantification
- Natera legacy & cell-free DNA
- Natera Core
- Natera's Innovations in Transplant Oncology
Prospera Transplant Assessment: Now Quantify Background cfDNA
Applying our expertise in over two million cfDNA tests has allowed us to refine risk of rejection in your transplant patients. Learn how the quantification of background cfDNA can provide more insights to you and your patients
Prospera: Quantifying background cfDNA to enhance performance
Prospera has now been enhanced with an exclusive technique, making it the first test of its kind to quantify absolute background cfDNA.
This enhancement benefits physicians by identifying patients with atypical background cfDNA levels -- thereby flagging them as at-risk for false-negative reporting and potentially missed rejections.
Dr. Gauthier introduces Prospera’s latest enhancement
Dr. Phil Gauthier, Natera Medical Director for Organ Transplantation, explains quantification of background cfDNA and its clinical impact on patient care
Why Background cfDNA Matters
Learn about the importance of background cfDNA levels when assessing your transplant patients for active rejection
View PDF
Evaluating patients with high background cfDNA
Insights and learnings from four patients where high background cfDNA levels were essential in assessing for active rejection
View PDF
Prospera: Quantifying background cfDNA to enhance performance
Read how quantifying background cell-free DNA (cfDNA) is raising the bar for precision in rejection assessment
View PDF
PEDAL Study: Prospera Enhancement by Detecting Background Cell-Free DNA Levels
View details of the PEDAL study and how quantification of background cell-free DNA allows for a more precise and confident assessment of allograft rejection.
View PDF
ASTS Webinar
Learn from Dr. Leeser about the latest research and enhancements of cfDNA testing, including the impact of quantifying background cfDNA when assessing for transplant rejection risk
Join Us
TTS 2020 Virtual Symposia - Impact of Background cfDNA in Renal Transplantation and Covid-19
Learn how the quantification of background cfDNA can provide more insights by assessing all types of kidney transplant rejection with greater precision, demonstrated by patient cases including Covid-19.
Prospera Transplant Assessment
Prospera is powered by highly optimized, proprietary cell-free DNA (cfDNA) technology. As part of your tool kit, Prospera assesses all types of kidney transplant rejection with great precision.

Prospera Physician Brochure
Learn about how the Prospera compares against first-generation cfDNA tests when assessing for active rejection.
View PDF
Prospera Patient Brochure
View our patient-facing brochure that highlights the importance of monitoring for rejection to preserve the function of a newly transplanted kidney with Prospera.
View PDF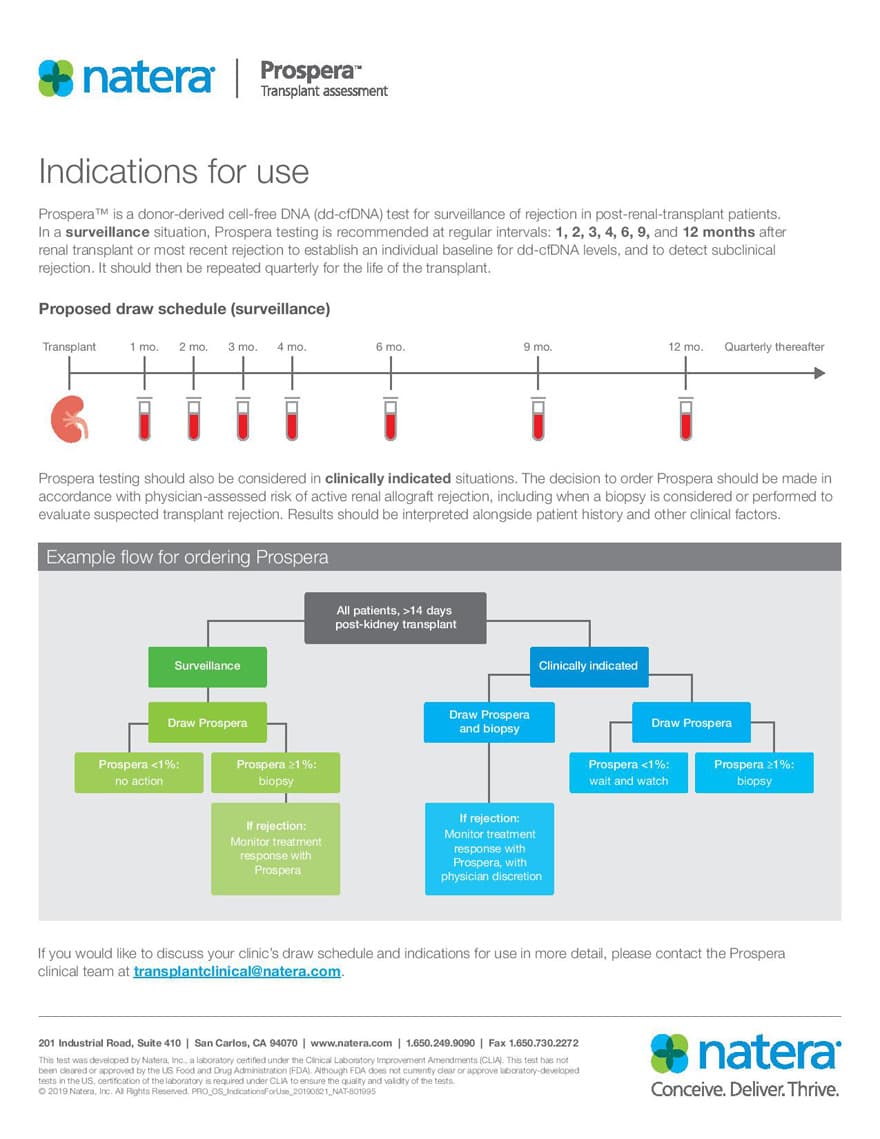
Prospera’s Indications for Use
See our proposed protocol for your transplant center. Prospera is recommended as a surveillance tool for rejection in renal transplant patients for all types of rejection.
View PDF
Prospera’s Guide to Results
Refer to this supplement to the Prospera report as a guide to your patient’s results.
View PDF
Highlights from our Clinical Validation Study
See the key takeaways from Sigdel et al, a clinical validation study for dd-cfDNA
View PDF
Prospera’s Clinical Validation Study
View the full clinical validation study: Sigdel et al Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR
View PDF
Prospera’s Analytical Validation Study
Click this link to view our clinical validation study on Prospera’s performance (Altug et al)
View PDF
Prospera’s Clinical Utility Study
Click this link to view our l clinical utility study and learn how Prospera helped change physician decision making (Peabody et al)
View PDFContact Us
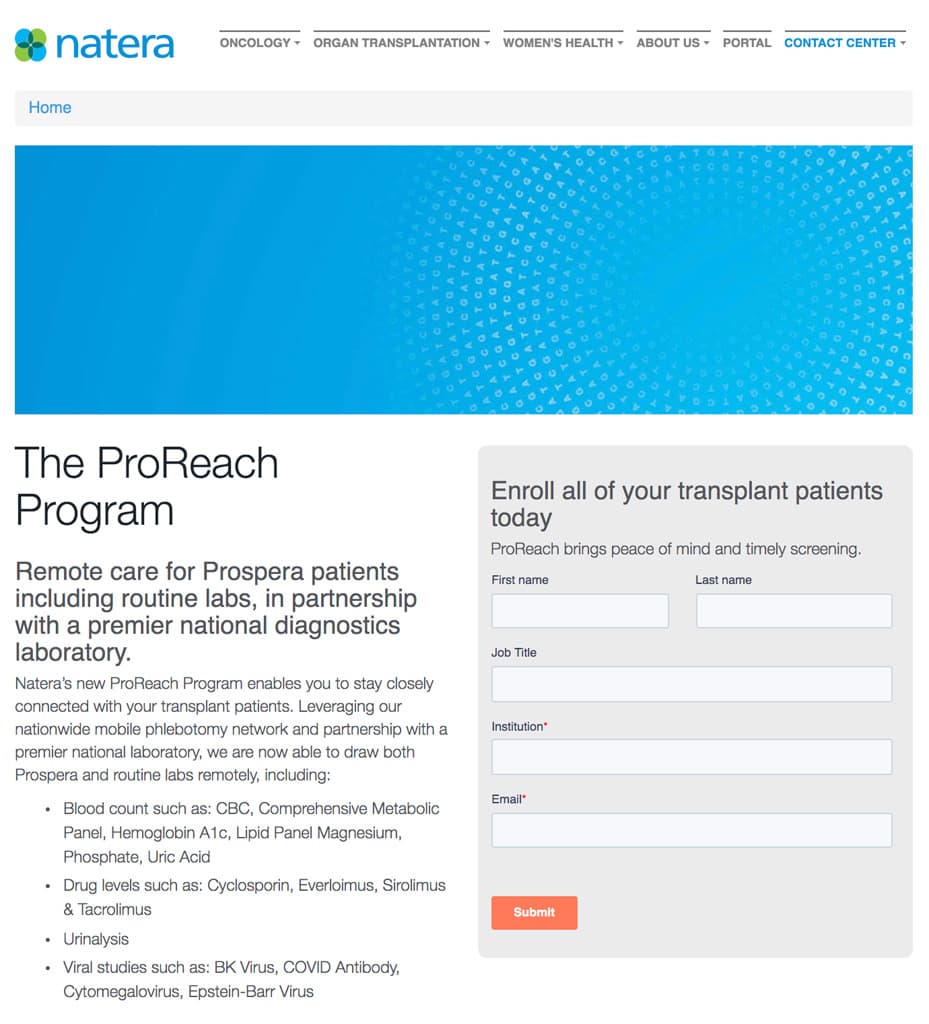
If you have any questions, reach out to us at ProsperaEvents@Natera.com
In the Prospera early access program, Natera has received tests from 45% of the top 50 and 37% of the top 100 transplant centers by volume
Second line
Natera May 4, 2020 Press Release