Accelerate kidney and rare disease drug development
The RenasightIQ™ clinicogenomic database empowers every stage of the drug development lifecycle with genetic, variant, and longitudinal clinical and claim data for over 215,000 chronic kidney disease (CKD) patients.
Built on the trusted clinical foundations of the Renasight™ kidney gene panel
Genetic data from 397 genes
Includes:
>12,700 Alport
>13,500 APOL1
>13,800 ADPKD
Variant-level data for over 200,000 CKD patients and growing
A clinical snapshot for >215,000 patients with CKD
Longitudinal clinical and claim data for each patient
De-identified patient dataset comprising of genetic findings, longitudinal claims, drug, and lab data including serum creatinine, eGFR, proteinuria and more.
With over 215,000 patients tested with Renasight™ and more patients being tested everyday, Natera’s ever growing database delivers real-world insights that can accelerate the drug development lifecycle. Pharma partners can leverage this data to make informed decisions in kidney disease research, ensuring faster timelines and improved patient outcomes.
Use RenasightIQ™ as a real-world evidence tool across the drug development lifecycle
1. Discovery & Preclinical Research
- Clinicogenomic datasets
- Patient cohorting and biomarker identification
- Real-world evidence for target validation
- Polygenic risk score insights (PRS)
- Drug Repurposing insights
2. Clinical Trials
- Clinicogenomic datasets
- Companion diagnostics
- Sponsored testing programs
- Real world comparator cohorts
- Longitudinal disease progression data
- Patient and site identification
3. Regulatory & Review Process
- Post-market surveillance and pharmacovigilance data
- Genomic subpopulation analysis
- Indication expansion opportunities
4. Commercial Launch
- HCP and patient marketing support
- Patient cohorting
- HEOR data
- Real-world utilization and adoption insights
- Disease and therapy education for HCPs
5. Post Market Safety Surveillance
- Genomic-driven pharmacovigilance
- Longitudinal patient tracking
- Subpopulation risk analysis
Natera’s kidney disease data services can help:
- Determine asset viability with real-world patient data
- Optimize clinical trial design for target recruitment
- Expedite clinical trial enrollment with precise population insights
- Understand the patient journey to identify gaps and opportunities
- Hone commercial targeting for effective therapy deployment
- Provide disease state and therapy education for stakeholders
- Enable patient journey mapping to improve outcomes

See the power of integrated genomic and clinical longitudinal data
RenasightIQ™ supports every stage in the drug development lifecycle. Through data licensing, analytics and clinical trial support services, Natera empowers Pharma innovators to better understand the kidney disease population and address unmet medical needs.
RenasightIQ™ Data Analytics and Licensing

Access and use RenasightIQ™ in clinical research within your organization and work directly with Natera’s Pharma Services team on descriptive, diagnostic, predictive and prescriptive analytics services.
Clinical Trial Services
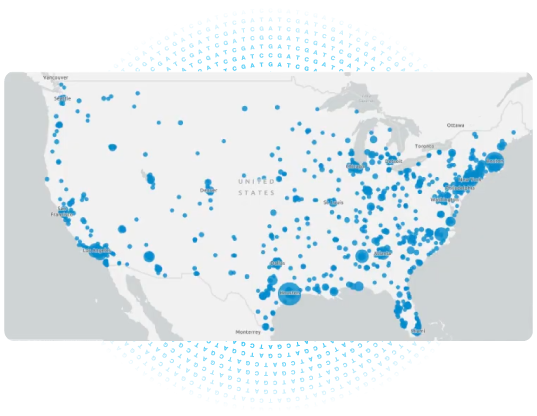
Leveraging RenasightIQ™ as the data foundation, Natera’s Pharma Services team offers TrialOptima™ to help optimize clinical trials through the following reports:
- Clinical population overview:
Characterize cohorts of interest by demographics, CKD stage, gene mutations, comorbidities, and geographic location. - Site ID:
Understand site and principal investigator viability through understanding the geographic location of patients who meet your trial criteria. - Trial patient matching:
Have Natera conduct outreach to patients who meet trial criteria determined by their Renasight™ test result and refer them to your trial.
Each of Natera’s partners benefit from direct access to a dedicated team of experts to assist in providing tailored insights and data synthesis to support your research goals. This team includes Bioinformaticians and Genetic Counselors who specialize in kidney disease.
Real-world outcomes with RenasightIQ™
Advancing Chronic Kidney Disease Precision Drug Development with Clinicogenomic Longitudinal Data
Genetic cohorting and therapeutic response in CKD: Real-world insights from RenasightIQ™
RenasightIQ™ was built for integration and collaboration
Uniquely focused on kidney disease
Leverages the Renasight™ test, a 397 gene panel that can determine the cause of one’s chronic kidney disease (CKD)
Clinical-grade accuracy
A CLIA-validated next generation sequencing (NGS) test
Largest commercially available kidney disease database
De-identified data for 200,000+ CKD patients
Data attributes proprietary to Natera
Information from test requisition forms as well as direct access to select patients and HCPs through Natera
Tailored Guidance
An expert data team comprised of bioinformaticians and genetic counselors specialized in kidney disease to assist with your data analysis
Natera uniquely combines expansive genetic datasets with longitudinal clinical data, creating unmatched precision and depth. Our end-to-end support from raw data licensing to actionable clinical insights help pharma innovators reduce costs, shorten timelines and enhance success rates in drug development.

