Natera’s Biopharma Services enable differentiated biomarker-guided trials to help bring life changing therapies to more patients, faster
Access the broadest populations
that may benefit from additional treatment
Expand therapeutic opportunities
in earlier disease settings
Support data-informed trial design
through integrated biomarker, real-world data, and AI-enabled insights
Accelerate clinical development
through earlier efficacy insights and streamlined trial execution
Our Advantage: Expand therapeutic opportunity to broader populations, sooner
Access broader patient populations
Expand your intent to treat populations or target populations with comprehensive biomarker stratification options
Enabled by: Molecular disease quantification, comprehensive genomic profiling, multi-omic assays, AI-enabled response signatures, and diverse RWD
Expand to earlier disease settings
Move drug development from metastatic to perioperative and adjuvant settings by identifying and enriching for higher-risk patients
Enabled by: Molecular disease quantitation to identify likely relapsers
Design more precise, data-informed trial designs
Predict response signatures, optimize trial design, refine inclusion/exclusion criteria, and simulate trial feasibility
Enabled by: Longitudinal RWD and AI-powered simulations and predictions
Execute trials faster
Predict therapy response as early as weeks into therapy initiation, accelerate recruitment, and scale seamlessly across geographies
Enabled by: Molecular disease monitoring, patient recruitment support, and global clinical trial and infrastructure
An integrated, comprehensive portfolio powering biomarker-guided development
Solutions Across the Drug Development Lifecycle
Delivering earlier insights for faster execution via a seamless partnership across the development lifecycle
Pre-clinical/Discovery
Which biomarkers and patient populations hold the greatest promise?
- Size and characterize addressable populations
- Discover AI-enabled, differentiated biomarkers
- Benchmark outcomes against standard of care
Early Clinical Development
How quickly can I see if my therapy is working, and in whom?
- Assess early efficacy within weeks using ctDNA dynamics
- Identify responder profiles and resistance mechanisms
- Define the optimal intent-to-treat population
Registrational Trial
How can I optimize trial design and accelerate execution?
- Optimize trial design and execution with AI-enabled simulations, ctDNA surrogate endpoints, and RWD comparator arms
- Accelerate recruitment by targeting biomarker-defined patient populations
- Scale globally through CDx commercialization and regulatory expertise
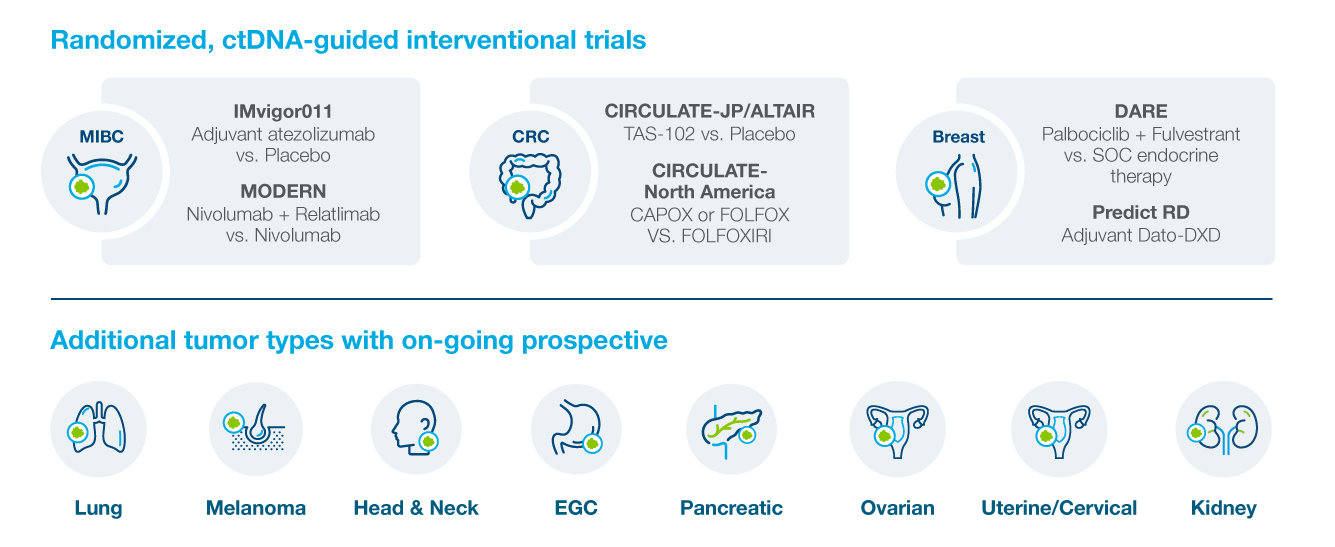
Multiple Prospective Interventional Studies
Combining Deep Scientific Expertise with Broad Commercial Reach
Natera integrates technology, evidence, and infrastructure to enable commercialization and trial execution at scale
Extensive Commercial Footprint1
400,000+ patients tested to date
40,000+ patients tested monthly
Deep Clinical Validation1
150+ Peer-reviewed publications across
30,000+ patients
30+ tumor types
Proven Biopharma Partnerships1
150+ prospective and retrospective studies
50+ Pharma partners
Global Reach & Access1
30+ Countries with clinical or commercial presence
4,500+ employees worldwide
US, Europe, Japan, China, and APAC operational infrastructure
Partnering for the Future of Oncology Drug Development
Natera enables differentiated, data-informed biomarker-guided trials to help bring therapies to more patients faster. Reach out to learn more.

How can Signatera™ support your clinical trials?
References
1Natera publicly reported and internal data as of January 2026.