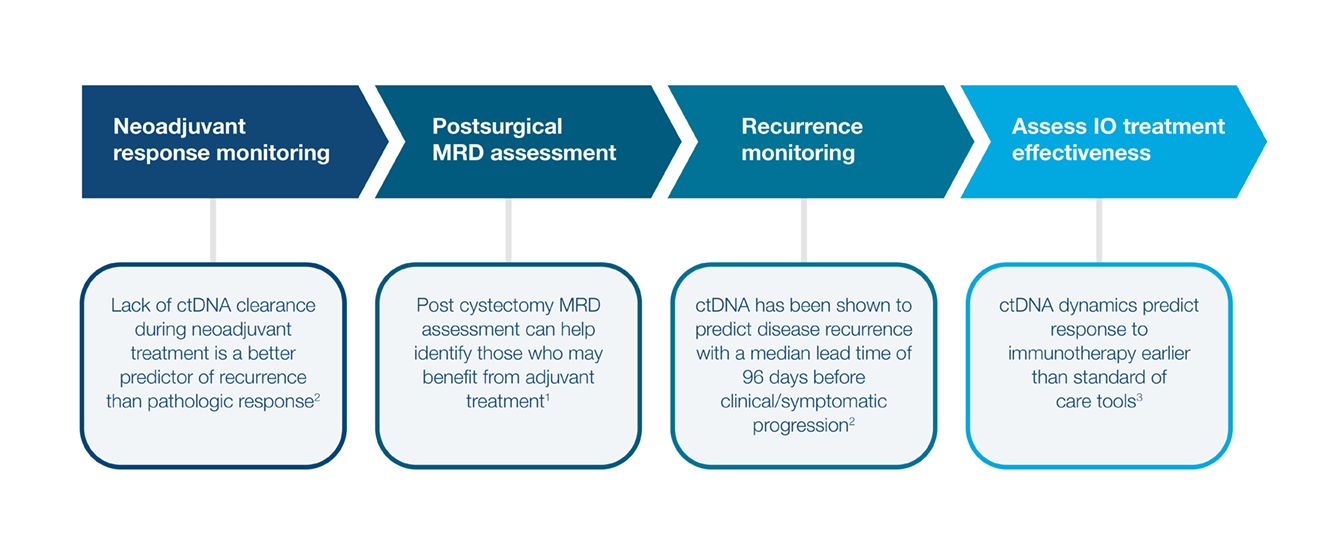
Integrating a ctDNA MRD-guided approach can enable more efficient trial design and de-risk development
Recently published Phase III IMvigor011 DFS and OS results (The New England Journal of Medicine, October 2025) highlight the feasibility and impact of MRD-guided enrollment in global, registrational-intent oncology trials.
MRD-guided enrichment reveals treatment effects obscured in all-comer trials
All-comer population (IMvigor010)
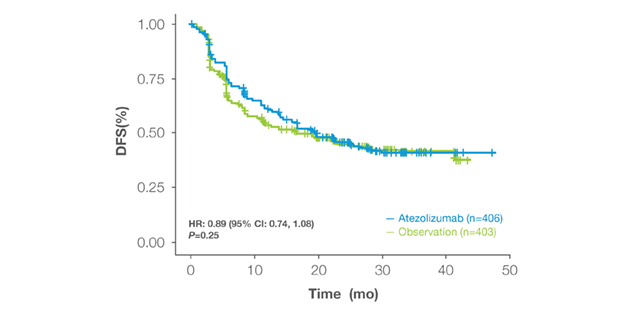
- IMvigor010: An all-comer adjuvant study that did not meet its primary DFS endpoint.1
- All-comer adjuvant trials that include low-risk, MRD-negative patients can dilute observed treatment effects, as many do well without therapy
MRD-guided enrichment can reveal treatment effects obscured in all-comer trials
ctDNA-positive population (IMvigor011)
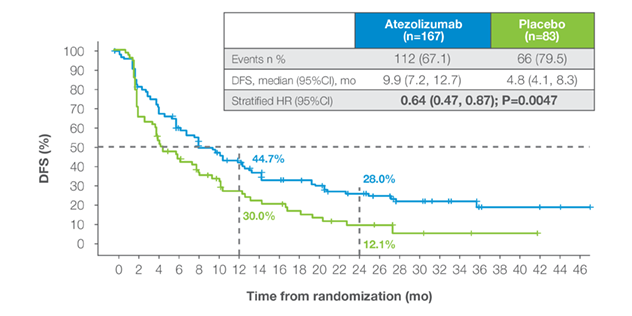
- IMvigor011: A Phase III trial that prospectively enrolled ctDNA-positive patients with improvements in DFS & OS in the treatment arm.6
- Prospective MRD-guided enrichment identifies higher-risk patients, yielding clearer therapy efficacy signals
MRD-guided enrollment can enable more efficient trial design and de-risks development
MRD status distinguishes high- and low-risk populations, enabling selection of optimal trial populations
ctDNA-positive post-cystectomy
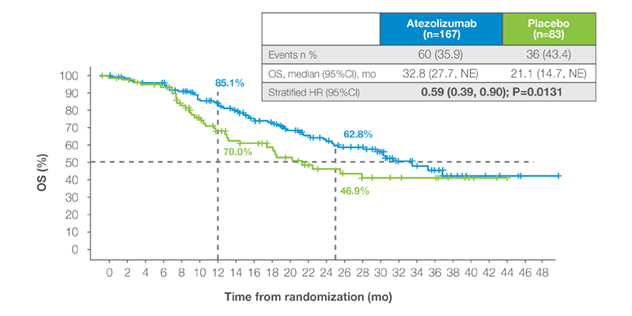
- ctDNA-positive patients showed significant DFS and OS benefit with adjuvant atezolizumab.6
- MRD-positive status defines a high-risk group where treatment effects are most evident.
By integrating MRD-guided enrichment, our pharma partners can design more efficient, focused trials with higher probability of success.
Persistently ctDNA-negative Post-cystectomy
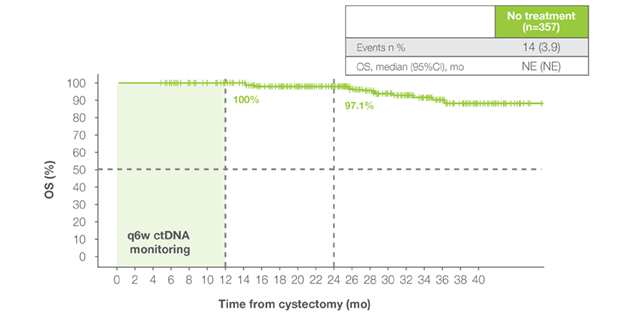
- Persistently ctDNA-negative patients demonstrated durable long-term outcomes without adjuvant therapy6
- Sustained MRD negativity identifies a low-risk population with minimal expected treatment benefit, and inclusion of these patients in adjuvant trials may dilute observed efficacy signals.
Persistently ctDNA-negative patients had excellent outcomes without adjuvant therapy – 100% OS at 12 months and 97.1% OS at 24 months post cystectomy.6
Now published in The New England Journal of Medicine
ctDNA-Guided Adjuvant Atezolizumab in Muscle-Invasive Bladder Cancer
Published October 20, 2025
Explore Previously Presented Signatera™ Results in MIBC
Signatera™ ctDNA positivity after cystectomy may predict adjuvant immunotherapy treatment benefit.1,4
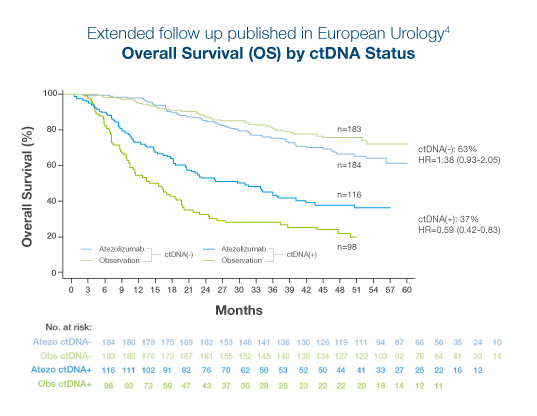
Extended follow-up from the Phase III, randomized IMvigor010 trial of atezolizumab vs observation in high risk adjuvant MIBC:4
- >110% survival benefit observed in ctDNA-positive patients treated with atezolizumab (OS, HR 0.59).4
- No treatment benefit was observed in ctDNA-negative patients treated with atezolizumab (OS, HR 1.38)4
- 37% of patients were ctDNA-positive at C1D1 and ctDNA positivity predicted benefit from immunotherapy at 46.8-month median follow-up (OS, HR=0.59)4
- >75% of patients with detectable ctDNA post-surgery in the observation arm recurred by 20 month follow up1
Natera is driving a paradigm shift for bladder cancer management and clinical trials across stages and therapeutic settings
Explore data across tumor types
How can Signatera™ support your clinical trials?
References
1Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432–437. DOI: 10.1038/s41586-021-03642-9.
2Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. Journal of Clinical Oncology. 2019;37(18):1547–1557. DOI: 10.1200/JCO.18.02052.
3Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1:873–881. DOI: 10.1038/s43018-020-0096-5.
4Powles T, et al. European Urology. 2023. DOI: 10.1016/j.eururo.2023.06.007.
5Powles T, et al. Presented at the EAU Annual Conference, 2024.
6Powles T, Kann AG, Castellano D, et al. IMvigor011: A phase 3 trial of ctDNA-guided adjuvant atezolizumab vs placebo in muscle-invasive bladder cancer. Presented at ESMO 2025 Congress, October 17–21, 2025, Berlin, Germany. Abstract LBA8. Concurrently published in New England Journal of Medicine. October 20, 2025. DOI: 10.1056/NEJMoa2511885.