Power Clinical Decisions in Gynecologic Cancer
Understand Risk
after completion of adjuvant/definitive therapy, ctDNA was detectable in
23%
of patients, all of whom experienced disease progression (HR: 17.6 95%CI: 3.2-97.4, p<0.001)1
Detect Recurrence Earlier
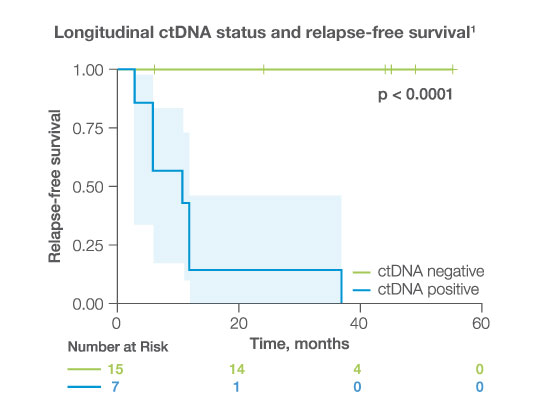
Longitudinally, ctDNA was detected with
100%
sensitivity and specificity while CA-125 had much lower sensitivity (43%) and specificity (78%)1,2
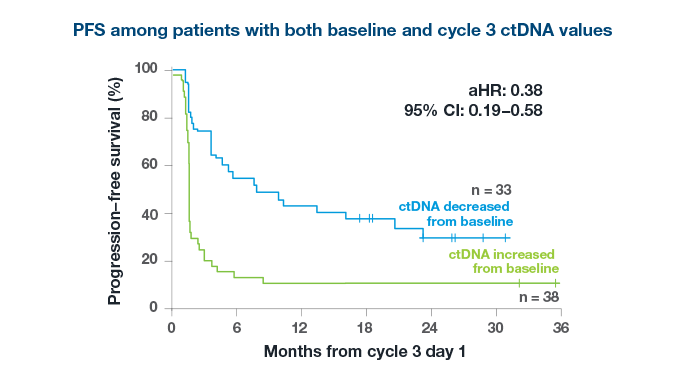
Predict Treatment Response
After just 2 cycles of immunotherapy
98%
of patients with an increase in ctDNA did not derive an objective response to treatment3*
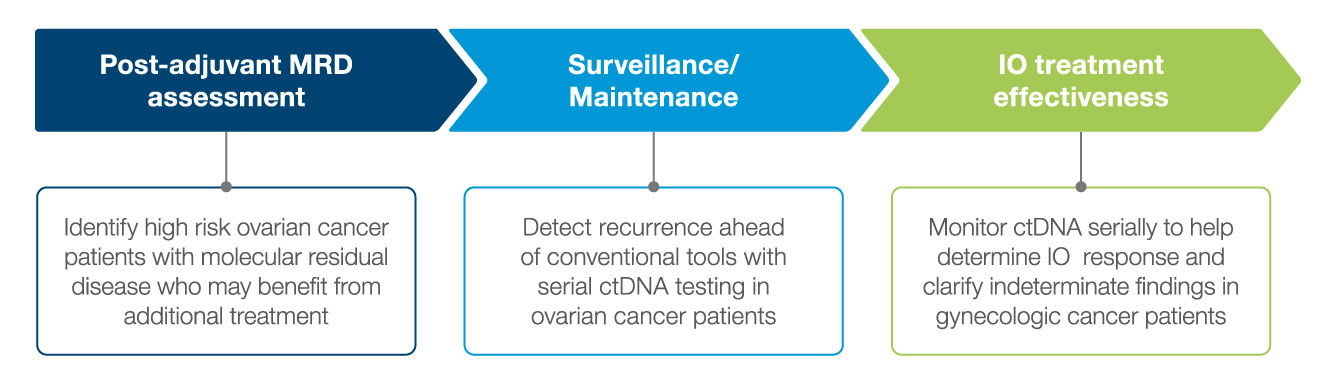
Actionable Molecular Insights Across the Patient Journey
Signatera™ enables early detection of molecular residual disease (MRD) to help you manage your patients with gynecologic cancers.
Discover the Data in Gynecologic Cancers
- Signatera™ is clinically validated cross multiple indications.
-
Post-Adjuvant MRD assessment
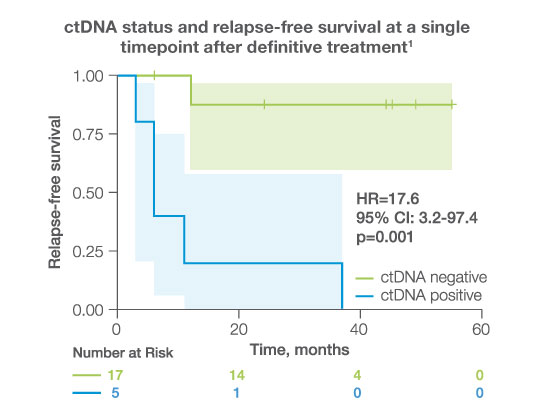
- After completion of adjuvant/definitive therapy, ctDNA was detectable in 23% of patients, all of whom experienced disease progression.1
-
Surveillance/Maintenance
-
Treatment Response Monitoring

Monitor Your Patients With Confidence
As part of her ovarian cancer monitoring plan, Anne received Signatera™ testing and CA-125 at regular intervals.
A positive Signatera™ result alerted her oncologist to order a scan, which caught a recurrence early. Now, Anne is receiving Signatera™ to monitor response to her maintenance therapy. Since starting therapy, Anne’s Signatera™ results have been negative, so she can feel more confident about her treatment plan.
Tailor Gynecologic Cancer Treatment With Natera’s Portfolio of Genomic Tests
| Signatera™ | Highly sensitive and perosnalized tumor-informed test for molecular residual disease (MRD) detection |
|---|---|
| Altera™ | Comprehensive genomic profiling for clinically relevant biomarkers that may help guide treatment selection (including MSI, BRCA1/2, HR genes, MMR genes, TMB, BRAF, RET, and NTRK), with no additional tumor sample needed. |
| Empower™ | Germline genetic test for commonly screened genes in gynecologic cancers (eg BRCA 1/2, MMR genes) to inform therapeutic decisions. |
More Clinician Resources
Signatera™ in Gynecologic Cancers
Read about the clinical utility of the Signatera™ test and data for MRD assessment, recurrence monitoring, and IO treatment response monitoring.

IO Monitoring Brochure
Learn how Signatera™ can assess immunotherapy response as early as 6 weeks into treatment for patients with ovarian, cervical, endometrial, and other cancers.2
Ready to try Signatera™ for your gynecologic cancer patients?
References
1Hou et al. Gynecol Oncol. 2022; 167:334-341.
2Chapman et al. Poster presented at 2021 AACR Annual Meeting.
3Bratman et al. Nature Cancer. 2020; 1(9):873-881.
Footnotes
*Includes high grade serous ovarian cancer (n=18), endometrial cancer (n=2), and cervical cancer (n=1).