Cancer tumor markers vs. Signatera circulating tumor DNA test
A deeper dive Into cancer biomarkers vs. Signatera™ circulating tumor DNA test
One of the most frequently asked questions we get is how Signatera is different from current biomarker (or tumor marker) tests. To answer this question, we will take a closer look at what tumor markers are, the common types used today, the differences between traditional biomarkers and Signatera, and how they work together to help doctors make better cancer care decisions.
Tumor marker tests were among the first available diagnostic tools for the management of cancer patients before the introduction of modern imaging techniques and the recent developments in molecular and cellular diagnostics. They continue to be widely used today, sometimes as a panel of multiple biomarkers or in combination with other tests, to indicate the presence of different types of cancer and provide important information about a cancer and the best way to treat it.
Alejandro Porto, CC BY-SA 3.0 , https://creativecommons.org/licenses/by-sa/3.0 via Wikimedia Commons
What is a biomarker? What is a tumor marker?
A biomarker (short for “biological marker”) is an objective measure that captures what is happening in a cell or an organism at a given moment. Biomarkers can serve as early warning systems for your health.
A tumor marker (also referred to as a cancer biomarker) is a molecule found in your bodily fluids or tissue that might indicate the presence of cancer. Tumor markers have traditionally been proteins or other molecules made by both the tumor and healthy cells in response to the tumor. However, genomic markers such as gene mutations, genetic patterns, and changes in the tumor DNA are now increasingly being used as tumor markers.1
There are three ways your doctor can test for tumor markers: a blood test (the most common method), a urine test, or a biopsy. Once the sample is collected, it is sent to a laboratory for analysis. You may need to repeat your tumor marker tests multiple times during and after treatment to determine if the treatment is working or whether there is a cancer recurrence, because your tumor marker levels can change over time.1,2
The type of test your doctor may recommend depends on various factors, such as your health, symptoms, and history. Also, there are different types of tumor markers for different types of cancer. For example:
| Biomarker | Utility1 |
|---|---|
| CA 125 (cancer antigen 125) | Used with ovarian cancer patients to determine if treatment is working and to detect cancer recurrence |
| CA 15-3 and CA 27-29 (cancer antigens 15-3 and 27-29) | Used to monitor treatment in people with advanced breast cancer |
| PSA (prostate-specific antigen) | Used to help diagnose prostate cancer, monitor treatment, and check to see if cancer has come back after treatment |
| CEA (carcinoembryonic antigen) | A marker for the presence of colorectal cancer (CRC), as well as for cancers of the lung, stomach, thyroid, pancreas, breast, and ovary; used to determine if treatment is working and to detect cancer recurrence |
| AFP (alpha-fetoprotein) | A marker for liver cancer and cancers of the ovary or testicles; used to help diagnose liver cancer, find out if cancer has spread, see if treatment is working, and predict chances for recovery2 |
Along with other tests, tumor marker tests can help doctors:
- diagnose specific types of cancer
- make decisions about the best therapy options
- monitor how well the treatment is working
- find out if a cancer has spread and make a prognosis of the disease outcome
- detect cancer recurrence after treatment
- screen people at high risk for cancer, including family history of cancer and previous diagnosis of another type of cancer2,3
Traditional cancer biomarkers can be very useful, the information they provide can also be limited. Some noncancerous conditions can lead to abnormal levels of tumor markers, and some people with cancer do not have trackable tumor markers. That is, the level of a tumor marker may not go up until after the cancer is advanced or has recurred. Also, not all types of cancer have related tumor markers. For these reasons, cancer biomarker tests are often combined with other diagnostic tests to help improve the accuracy of results.2,3
What is the Signatera circulating tumor DNA test?
Unlike biomarker tests that measure specific proteins, such as prostate-specific antigen (PSA) or carcinoembryonic antigen (CEA), Signatera detects very small traces of circulating tumor DNA (ctDNA) in your blood. The Signatera test is a personalized, tumor-informed assay that is custom-built to your unique set of tumor mutations. To create the personalized assay, the DNA from the tumor and normal cells are sequenced and compared to isolate the genetic alterations that are only present in your tumor. This enables Signatera to then be extremely effective at detecting the presence of a very small amount of tumor DNA or remaining cancer in the body during or after treatment (referred to as “molecular residual disease,” or MRD).
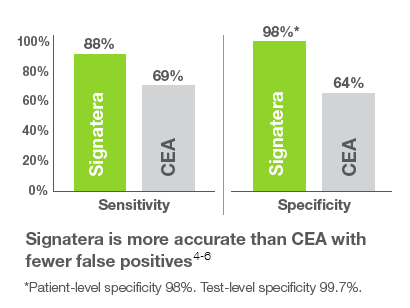
Recent studies in colorectal cancer (CRC) patients have shown that Signatera is more accurate than CEA, predicting cancer recurrence with 88% sensitivity (that is, it is able to correctly identify people who have the disease) and 98% specificity (meaning it is also able to correctly identify people who do not have the disease, or false positives).4-6
Doctors use Signatera to:
- establish a baseline after initial cancer diagnosis
- determine if additional therapy may be needed after initial treatment
- monitor for MRD to evaluate how well the treatment is working
- detect cancer recurrence earlier than some other methods
A negative Signatera result indicates that tumor DNA was not detected in your blood and risk for recurrence is reduced. If you have a positive result, there is a 98% chance that your cancer has returned.4,7-9
The bottom line is, Signatera is a tumor DNA specific biomarker used in a very specific context of molecular residual disease detection, and cancer and treatment response monitoring. While traditional biomarkers are utilized for other applications that include cancer screening, therapy selection, and clinical trial match.
How Signatera and CEA work together for early cancer relapse detection
Signatera is meant to be ordered regularly during treatment and follow-up care to look for MRD. To improve patient care and quality of life, it can be used alongside CEA to detect CRC recurrence earlier—while it’s easier to treat—or to reduce false positive CEA results.
Additional resources
References
1Tumor Markers. National Cancer Institute. Updated May 6, 2019. Accessed September 23, 2020. https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-markers-fact-sheet
2Tumor Marker Tests. MedlinePlus. Updated July 31, 2020. Accessed September 23, 2020. https://medlineplus.gov/lab-tests/tumor-marker-tests/
3Hibbs S. What are tumor marker tests for cancer? 8 things you need to know. Cancer.net. September 17, 2019. Accessed September 23, 2020. https://www.cancer.net/blog/2019-09/what-are-tumor-marker-tests-cancer-8-things-you-need-know
4Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124-1131.
5Purandare NC, Dua SG, Arora A, Shah S, Rangarajan V. Colorectal cancer — patterns of locoregional recurrence and distant metastases as demonstrated by FDG PET/CT. Indian J Radiol Imaging. 2010;20(4):284-288.
6Advance Staff. Colorectal cancer monitoring. Elite Healthcare. 2016;25(9):14. Accessed September 23, 2020. https://www.elitecme.com/resource-center/laboratory/colorectal-cancer-monitoring/
7Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
8Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017,545(7655):446-451.
9Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. 2019; 37(18):1547-1557.